Chapter 6.1 - Radioactivity Exercise 261
Question 1
Name the three elementary particles present in an atom. Locate their positions.
Solution 1
Atom consists of three elementary particles, neutrons, protons and electrons. Neutron and protons form the central part of atom called nucleus where electrons revolve around this central part in orbits called electronic orbits.
Question 2
Solution 2

Question 3
Solution 3

Question 4
Solution 4

Question 5
A radioactive source emits three types of radiations. Name them.
(i) Name the radiations which are charged.
(ii) Name the radiations which are most penetrating.
(iii) Name the radiations which travel with the speed of light.
(iv) Name the radiations which have the largest mass.
(i) Name the radiations which are charged.
(ii) Name the radiations which are most penetrating.
(iii) Name the radiations which travel with the speed of light.
(iv) Name the radiations which have the largest mass.
Solution 5

Question 6
'Radioactivity is a nuclear phenomenon'. Comment on this statement.
Solution 6
Radioactivity is the spontaneous random emission of particles from within the nucleus of atom. Radiations are emitted from nucleus of atom thus radioactivity is a nuclear phenomenon.
Question 7
Solution 7

Question 8
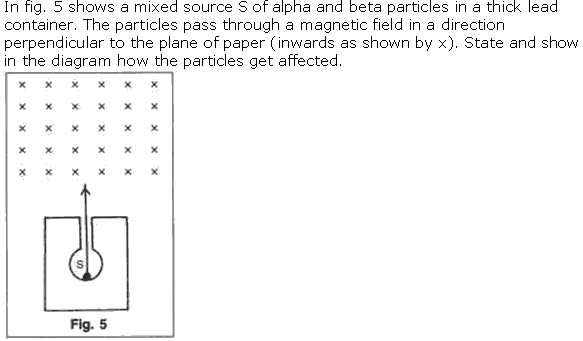
Solution 8

(i) Alpha particles are heavy in mass and are positively charged so they are deflected less by magnetic field and direction is upward which can be calculated by Fleming left hand rule.
(ii) Beta particles are negligible in mass so they are highly deflected by magnetic field and they are negatively charge particle so they are deflected in downward direction.
(iii) Gamma radiations have no mass and no charge so they are not deflected by magnetic field.
Question 9
Define nucleus.
Solution 9
Nucleus is central part of an atom which consist of elementary particles protons and neutrons.
Question 10
What do you mean by (i) Atomic number, (ii) Mass number, (iii) Atomic mass
Solution 10
(i) Atomic number is the number of protons present in the nucleus. As number of protons is equal to the number of electrons so atomic number also gives the number of electrons in an atom.
(ii) Mass number is the sum of protons and neutrons present in the nucleus.
(iii) Atomic mass of element is the relative mass of its atom as compared to the mass of carbon atom taken as 12.
(ii) Mass number is the sum of protons and neutrons present in the nucleus.
(iii) Atomic mass of element is the relative mass of its atom as compared to the mass of carbon atom taken as 12.
Question 11
Solution 11

Question 12
Solution 12
Question 13
What are isotopes?
Solution 13
The atoms of same elements having the same atomic number Z but different mass number A are called isotopes.
Question 14
Do isotopes have same chemical or physical properties?
Solution 14
Isotopes have same chemical properties but have different physical properties.
Question 15
What are isobars?
Solution 15
The atoms of different elements having same mass number but different atomic number are called isobars.
Question 16
Solution 16
Similarities:
Both ?-radiations and X-rays affect photographic plate, both travel with the speed of light.
Dissimilarities:
?-radiations are obtained in emissions from the radioactive substances due to energy change in the nucleus of their atoms and X- rays are obtained when highly energetic cathode rays are stopped by a heavy metal target of high melting point.
?-radiations have high penetration power but X-rays do not have have very high penetration power.
Both ?-radiations and X-rays affect photographic plate, both travel with the speed of light.
Dissimilarities:
?-radiations are obtained in emissions from the radioactive substances due to energy change in the nucleus of their atoms and X- rays are obtained when highly energetic cathode rays are stopped by a heavy metal target of high melting point.
?-radiations have high penetration power but X-rays do not have have very high penetration power.
Chapter 6.1 - Radioactivity Exercise 262
Question 1

Solution 1

Question 2
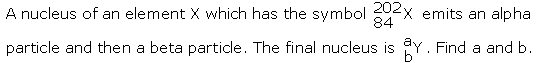
Solution 2

Question 3
The mass number (A) of an element is not changed when it emits ______________.
Solution 3
Question 4
The atomic number of a radioactive element is not changed when it emits ............
Solution 4
Question 5

Solution 5

Question 6
What are radioisotopes? State two uses of radioisotopes.
Solution 6
Artificial radioactive substances can be produced by bombarding lighter nuclides with alpha particles, protons and neutron. The radioactive substances produces in this manner are called radioisotopes.
Radioisotopes can be used as:
(i) Rays from Radium produce satisfactorily improvement in skin diseases.
(ii) Radioactive Sulphur S35 helps to study advantages and disadvantages of fungicides.
Radioisotopes can be used as:
(i) Rays from Radium produce satisfactorily improvement in skin diseases.
(ii) Radioactive Sulphur S35 helps to study advantages and disadvantages of fungicides.
Question 7
State three safety precautions that you would take while handling the radioactive substances.
Solution 7
Following precautions should be taken while handling the radioactive substances.
(i) The sources should only be handled by the forceps provided and never touched by hand.
(ii) They should never be pointed towards a person.
(iii) Food should not be taken where the sources are being used, as it may be contaminated.
(iv) Never smoke near a radioactive source.
(i) The sources should only be handled by the forceps provided and never touched by hand.
(ii) They should never be pointed towards a person.
(iii) Food should not be taken where the sources are being used, as it may be contaminated.
(iv) Never smoke near a radioactive source.
Question 8
Why should a radioactive substance not be touched by hand?
Solution 8
Radioactive substances should not be touched by hands because radiation emitted by radioactive substances can cause burns, Leukaemia, eye cataract, sterility or many other dangerous disease.

0 comments:
Post a Comment