Chapter 6 - Modern Physics - Exercises and MCQ Exercise 266
Question 1
Make the correct choices in the following:
(a) In one atom of copper (mass number 63, atomic number 29) there are
(i) 63 protons and 29 neutrons.
(ii) 63 protons and 29 electrons.
(iii) 29 protons and 29 neutrons.
(iv) 29 protons and 29 electrons.
(v) 29 protons and 63 neutrons.
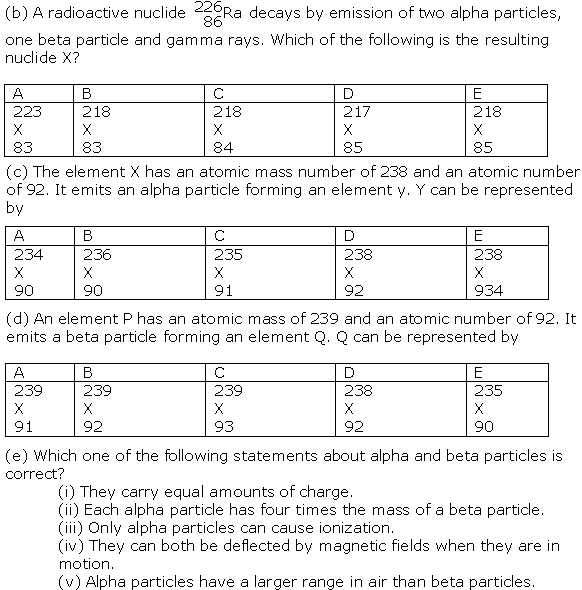
(a) In one atom of copper (mass number 63, atomic number 29) there are
(i) 63 protons and 29 neutrons.
(ii) 63 protons and 29 electrons.
(iii) 29 protons and 29 neutrons.
(iv) 29 protons and 29 electrons.
(v) 29 protons and 63 neutrons.

Solution 1
(a) Mass number of copper = 63
Atomic number of copper = 29
As atomic number of element gives number of proton and electrons while mass number of element gives number of protons +number of neutrons.
So, number of protons in copper = atomic number of copper = 29.
Number of electron in copper = number of protons in copper =29.
Number of neutrons = mass number atomic number = 63 29 =34.
So answer is (iii) as copper contains 29 protons and 29 electrons.

Atomic number of copper = 29
As atomic number of element gives number of proton and electrons while mass number of element gives number of protons +number of neutrons.
So, number of protons in copper = atomic number of copper = 29.
Number of electron in copper = number of protons in copper =29.
Number of neutrons = mass number atomic number = 63 29 =34.
So answer is (iii) as copper contains 29 protons and 29 electrons.

Chapter 6 - Modern Physics - Exercises and MCQ Exercise 267
Question 1
Solution 1
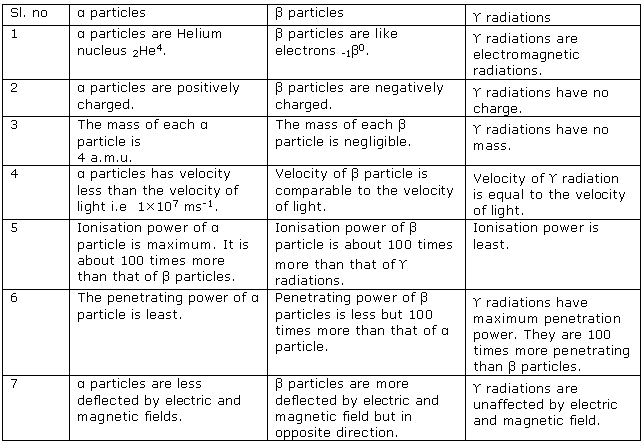
Question 2
What do you understand by radioactive decay? An isotope of Uranium is radioactive and changes into Thorium 92 by the emission of an alpha particle. Write a symbolic equation for this decay process.
is radioactive and changes into Thorium 92 by the emission of an alpha particle. Write a symbolic equation for this decay process.
Solution 2
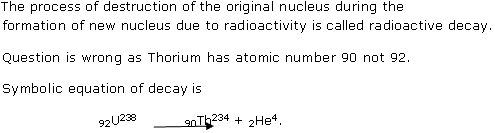
Question 3
The element  changes to magnesium by the emission of a beta particle. Write down the symbolic equation for the beta emission.
changes to magnesium by the emission of a beta particle. Write down the symbolic equation for the beta emission.
Solution 3

Question 4
What are the uses of radioactive isotopes in following?
(a) Medical field,
(b) Agriculture,
(c) Industries?
(a) Medical field,
(b) Agriculture,
(c) Industries?
Solution 4

Question 5
What do you mean by background radiations? Name its sources. Is it possible for us to keep ourselves away from it?
Solution 5
The low temperature microwave radiation that arrives at the earth's surface from all directions of outer space is called background radiation.
Sources of background radiations are:
(a) Radiation from the sun.
(b) Rocks in the earth which contain traces of radioactive substances.
(c) Naturally occurring isotopes.
(d) Artificial radioisotopes.
No, it is not possible to keep ourselves away from these radiations.
Sources of background radiations are:
(a) Radiation from the sun.
(b) Rocks in the earth which contain traces of radioactive substances.
(c) Naturally occurring isotopes.
(d) Artificial radioisotopes.
No, it is not possible to keep ourselves away from these radiations.
Question 6
State some of the safety precautions that we have to taken when we are exposed to some of the radiations or when we are handling some of the radioactive substances.
Solution 6
Following precautions should be taken while handling the radioactive substances.
(i) The sources should only be handled by the forceps provided and never touched by hand.
(ii) They should never be pointed towards a person.
(iii) Food should not be taken where the sources are being used, as it may be contaminated.
(iv) Never smoke near a radioactive source.
(i) The sources should only be handled by the forceps provided and never touched by hand.
(ii) They should never be pointed towards a person.
(iii) Food should not be taken where the sources are being used, as it may be contaminated.
(iv) Never smoke near a radioactive source.
Question 7
Define the term radioactivity. Of the three normally occurring radioactive radiations:
(a) Which has a positive charge?
(b) Which is most penetrating?
(c) Which has no electric charge?
(a) Which has a positive charge?
(b) Which is most penetrating?
(c) Which has no electric charge?
Solution 7

Question 8
Which of the following statements are correct?
(a) Electrons have
(i) No mass,
(ii) A mass less than that of a proton,
(iii) A mass equal to that of a helium atom,
(iv) Mass greater than that of a neutron.
(b) Neutrons have
(i) A double positive charge,
(ii) A single positive charge,
(iii) A negative charge,
(iv) No electric charge.
(a) Electrons have
(i) No mass,
(ii) A mass less than that of a proton,
(iii) A mass equal to that of a helium atom,
(iv) Mass greater than that of a neutron.
(b) Neutrons have
(i) A double positive charge,
(ii) A single positive charge,
(iii) A negative charge,
(iv) No electric charge.
Solution 8
(a) Electron has (ii) a mass less than that of a proton.
(b) Neutrons have (iv) no electric charge.
(b) Neutrons have (iv) no electric charge.
Question 9
Draw a diagram of a simple atom showing the nucleus and electrons. In this atom:
(a) What type of charge will there be on the nucleus?
(b) What is the value of this charge?
(a) What type of charge will there be on the nucleus?
(b) What is the value of this charge?
Solution 9
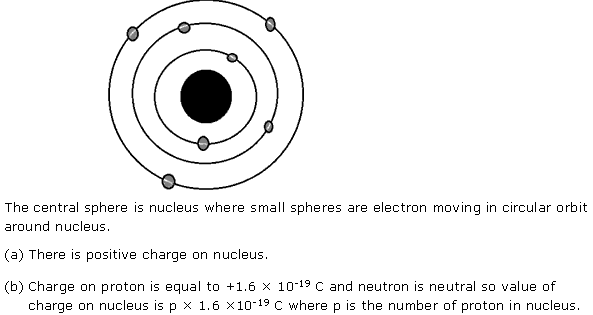
Question 10
What are the names of the three types of rays given off by a radioactive substance? Define the properties you know they possess. Why are the largest atoms radioactive?
Solution 10

Question 11
One isotopes of uranium has a mass number of 235 and atomic number 92.
(a) What is the number of electrons in a neutral atom of this isotope?
(b) How many protons are there in the nucleus of this isotope?
(c) For another isotope of Uranium state which one of the numbers (238 or 92) changes?
(d) What is the number of protons in 238U?
(a) What is the number of electrons in a neutral atom of this isotope?
(b) How many protons are there in the nucleus of this isotope?
(c) For another isotope of Uranium state which one of the numbers (238 or 92) changes?
(d) What is the number of protons in 238U?
Solution 11
(a)Mass number of uranium = 235
Atomic number of uranium = 92
As atomic number of element gives number of proton and electrons while mass number of element gives number of protons +number of neutrons.
So, number of protons in uranium = atomic number of uranium = 92.
(b) Number of electron in uranium = number of protons in uranium =92.
(c)The atoms of same elements having the same atomic number Z but different mass number A are called isotopes. So, for another isotope of uranium mass number 235 changes.
(d)Number of protons in isotopes is same and as U238 is isotope of 92U235. So, number of protons in U235 is also 92.
Atomic number of uranium = 92
As atomic number of element gives number of proton and electrons while mass number of element gives number of protons +number of neutrons.
So, number of protons in uranium = atomic number of uranium = 92.
(b) Number of electron in uranium = number of protons in uranium =92.
(c)The atoms of same elements having the same atomic number Z but different mass number A are called isotopes. So, for another isotope of uranium mass number 235 changes.
(d)Number of protons in isotopes is same and as U238 is isotope of 92U235. So, number of protons in U235 is also 92.
Question 12
Radioactive substances were found to give off three types of rays. Name them. How do they
(a) React to the magnetic field?
(b) React to the electric field?
(c) Act when different thickness of lead sheets is placed in their path?
(a) React to the magnetic field?
(b) React to the electric field?
(c) Act when different thickness of lead sheets is placed in their path?
Solution 12

Question 13
Chlorine has an atomic number 17 in the form of isotope, one of atomic weight 35 and the other atomic weight 37. What do you understand by an isotope? Name two other elements which have isotopes.
Solution 13
The atoms of same elements having the same atomic number Z but different mass number a are called isotopes. Hydrogen have 3 isotopes protium 1H1, deuterium 1H2, tritium 1H3.
Question 14
(a) What is the meaning of mass number?
(b) What do you mean by atomic number?
(c) In what way are these numbers related to the isotopes of an element?
(d) Explain the use of radioactive in the field of medicine, agriculture and industry.
(b) What do you mean by atomic number?
(c) In what way are these numbers related to the isotopes of an element?
(d) Explain the use of radioactive in the field of medicine, agriculture and industry.
Solution 14


0 comments:
Post a Comment