Chapter 5 - Heat - Exercises and MCQ Exercise 245
Question 1
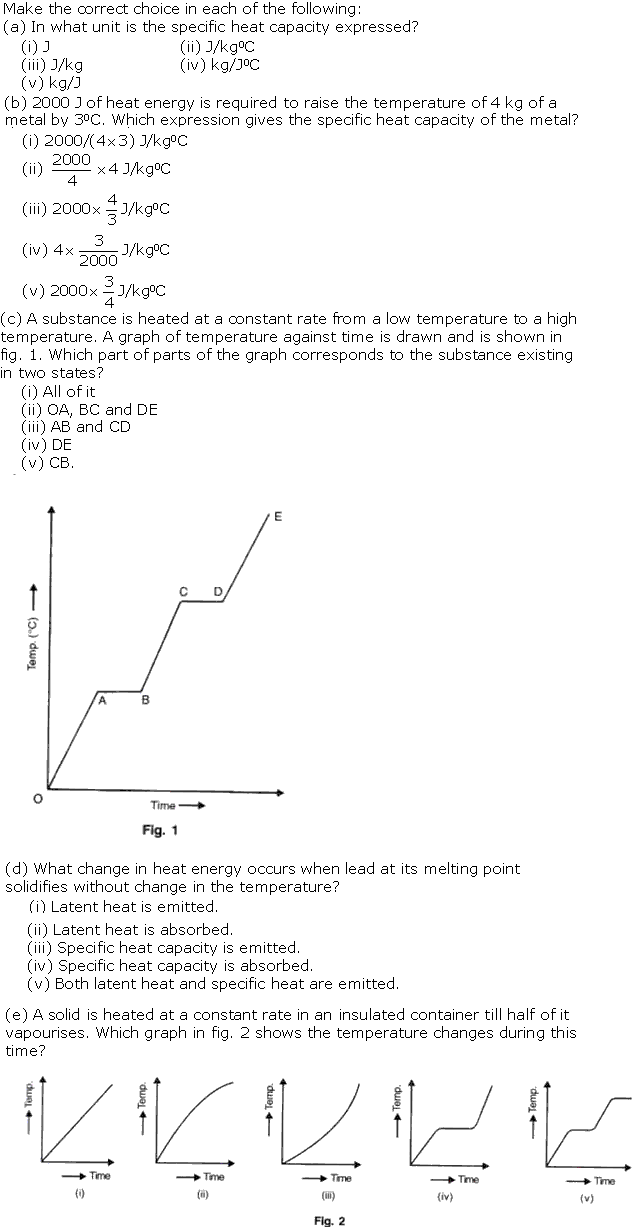
Solution 1
(a)J/kg oC
(b)2000 / (4 x 3) J/kg oC
(c)AB and CD
(d)Latent heat is emitted
(e)The first time when temperature is constant represents change of state from solid to liquid and the second time temperature is constant represents change of state from liquid to vapour.
(b)2000 / (4 x 3) J/kg oC
(c)AB and CD
(d)Latent heat is emitted
(e)The first time when temperature is constant represents change of state from solid to liquid and the second time temperature is constant represents change of state from liquid to vapour.
Chapter 5 - Heat - Exercises and MCQ Exercise 246
Question 1
(a) Define the following terms:
(i) Specific latent heat,
(ii) Specific latent heat of fusion.
(b) What do you understand by the statement sp. latent heat of vapourisation of steam is 2268 J/kg?
(c) Calculate the amount of heat given out while converting 100 g of water at 50oC into ice at -5oC. (refer Q.9 for data).
(i) Specific latent heat,
(ii) Specific latent heat of fusion.
(b) What do you understand by the statement sp. latent heat of vapourisation of steam is 2268 J/kg?
(c) Calculate the amount of heat given out while converting 100 g of water at 50oC into ice at -5oC. (refer Q.9 for data).
Solution 1
Question 2
When steam at 100oC is passed through a hole drilled in a slab of ice at 0oC certain amount of ice melts. Find the amount of ice that melts. (sp. latent heat of fusion of ice = 336 J/g, sp. latent heat of vapourisation of steam = 2268 J/g, Sp. heat cap. of water= 4.2 J/goC) (Take mass of steam as 1000 g)
Solution 2
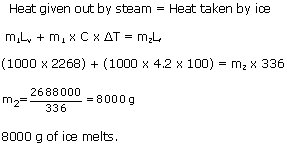
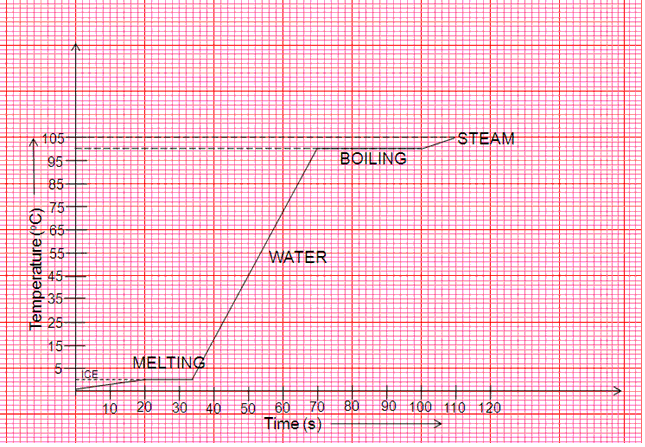
Question 3
A slab of ice at -5oC is constantly heated till it changes to steam at 105oC. Draw a graph showing the change of temperature with time and label the different parts of the graph.
Solution 3
Question 4
200 g of of ice at 0oC is added to 2 kg of water at 30oC. What is the temperature of the mixture?
Solution 4
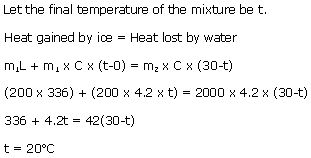
Question 5
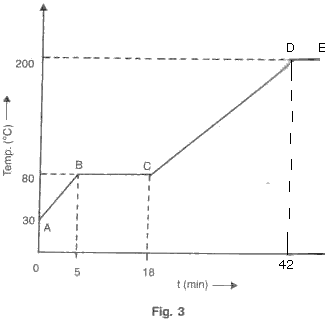
A solid of mass 100 g is heated with a burner which is supplying heat at the rate of 100 J/s. The graph in fig. 3 shows the changes that take place.
(a) What is the melting point of the substance?
(b) What is boiling point of the substance?
(c) Calculate the Sp. heat cap. of solid.
(d) Calculate the Sp. latent heat of fusion.
(e) Calculate the Sp. heat cap. of the liquid.
(a) What is the melting point of the substance?
(b) What is boiling point of the substance?
(c) Calculate the Sp. heat cap. of solid.
(d) Calculate the Sp. latent heat of fusion.
(e) Calculate the Sp. heat cap. of the liquid.

Solution 5

Question 6
(a) Define heat capacity and state its units.
(b) What amount of heat would be required to raise the temperature of 500 g of water from 30oC to 50oC? (sp. heat cap. of water= 4200 J/kgoC)
(b) What amount of heat would be required to raise the temperature of 500 g of water from 30oC to 50oC? (sp. heat cap. of water= 4200 J/kgoC)
Solution 6
(a)Heat capacity of a body is the quantity of heat required to raise its temperature by 1oC. It depends upon the mass and the nature of the body.
Units: J/oC or calorie/oC
(b)Change in temperature = (50-30) = 20oC
Amount of heat required, Q = m x C x ?T
= 0.5 x 4200 x 20 = 42000 J
Units: J/oC or calorie/oC
(b)Change in temperature = (50-30) = 20oC
Amount of heat required, Q = m x C x ?T
= 0.5 x 4200 x 20 = 42000 J
Question 7
(a) Sp. heat capacity of copper is 390 J/kgoC. What do you understand by this statement?
(b) Calculate the amount of heat given out by 600 g of aluminium while cooling from 100oC to 30oC. (Sp. heat cap. of aluminium = 900 J/kgK.)
(b) Calculate the amount of heat given out by 600 g of aluminium while cooling from 100oC to 30oC. (Sp. heat cap. of aluminium = 900 J/kgK.)
Solution 7
(a)This means that 390 J of heat is required to raise the temperature of 1kg of copper by 1oC.
(b)Change in temperature = (100-30) = 70oC = 70 K
Amount of heat given out, Q = m x C x T
= 0.6 x 900 x 70 = 37800 J
(b)Change in temperature = (100-30) = 70oC = 70 K
Amount of heat given out, Q = m x C x T
= 0.6 x 900 x 70 = 37800 J
Question 8
(a) State the principle of calorimeter and define calorie and kilocalorie.
(b) 400 g of water at 80oC is mixed with 1 kg of water at 20oC. If the sp. heat cap. of water is 4200 J/kgK, find the final temperature of the mixture.
(c) A burner raises the temperature of 360 g of water from 40oC to 100oC in 5 minutes. Calculate the rate of heat supplied by the burner.
(b) 400 g of water at 80oC is mixed with 1 kg of water at 20oC. If the sp. heat cap. of water is 4200 J/kgK, find the final temperature of the mixture.
(c) A burner raises the temperature of 360 g of water from 40oC to 100oC in 5 minutes. Calculate the rate of heat supplied by the burner.
Solution 8
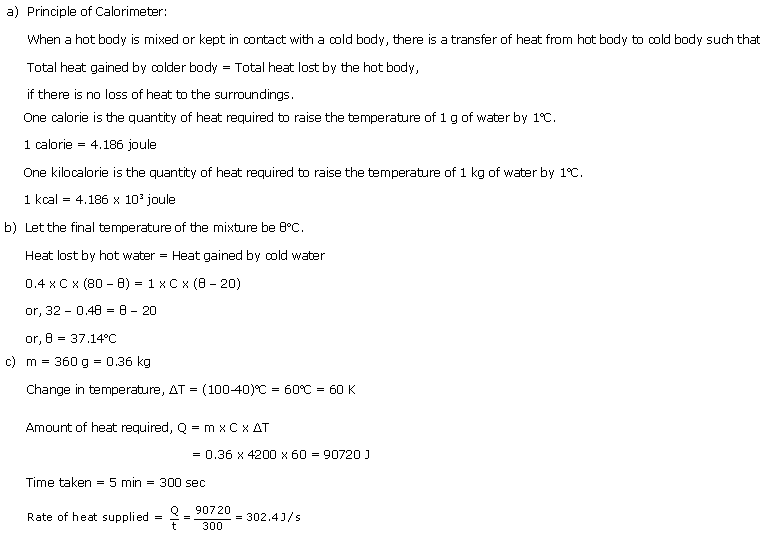
Question 9
A solid of mass 80 g at 80oC is dropped in 400 g water at 10oC. If final temp. is 30oC, find the sp. heat cap. of the solid.
Solution 9
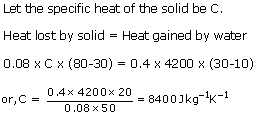
Question 10
A mass of 200 g of mercury at 100oC is poured into water at 20oC. If the final temperature of the mixture is 25oC, find the mass of water. (Sp. heat cap. of mercury is 140 J/kgK).
Solution 10
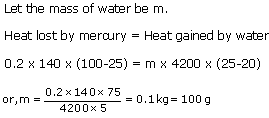
Question 11
A piece of copper of mass 1 kg is dropped into 2 kg of water at 15oC. If the final temperature of the mixture is 40oC, calculate the intial temperature of copper.
Solution 11
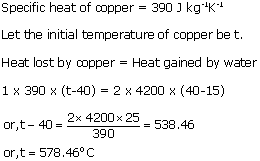
Question 12
A copper calorimeter of heat capacity 32 J/oC contains 100 g of oil at 30oC. 80 g of a metal of sp. heat cap. 0.12 J/goC at 90oC is dropped into the oil and final temperature is 35oC. Calculate the sp. heat cap. of the oil.
Solution 12

Question 13
(a) Define the following terms:
(i) Latent heat,
(ii) Latent heat of fusion of ice.
(b) Calculate the amount of heat required to change 40 G of ice at -10oC into water at 20oC. (sp. heat cap. of ice = 2.1 J/goC, latent heat of fusion of ice = 330 j/g)
(i) Latent heat,
(ii) Latent heat of fusion of ice.
(b) Calculate the amount of heat required to change 40 G of ice at -10oC into water at 20oC. (sp. heat cap. of ice = 2.1 J/goC, latent heat of fusion of ice = 330 j/g)
Solution 13
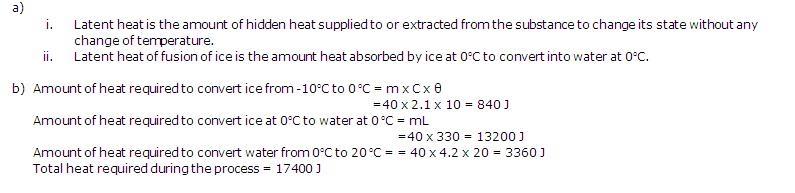
Chapter 5 - Heat - Exercises and MCQ Exercise 247
Question 1
1 kg of molten lead at its melting point of 327oC is dropped in 1 kg of water at 20oC. Assuming no heat is lost; calculate the final temperature of water. (Sp. heat cap. of lead = 130 J/kgoC, Sp. latent heat of fusion of lead = 27000 J/g, Sp. heat cap. of water = 4200 j/kgoC)
Solution 1
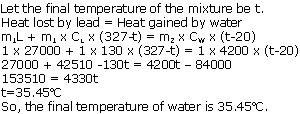
Question 2
How much steam at 100oC must be passed into 120 g of water at 20oC to raise the temperature to 40oC? (Use data from Q.11)
Solution 2
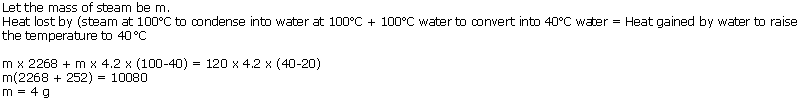
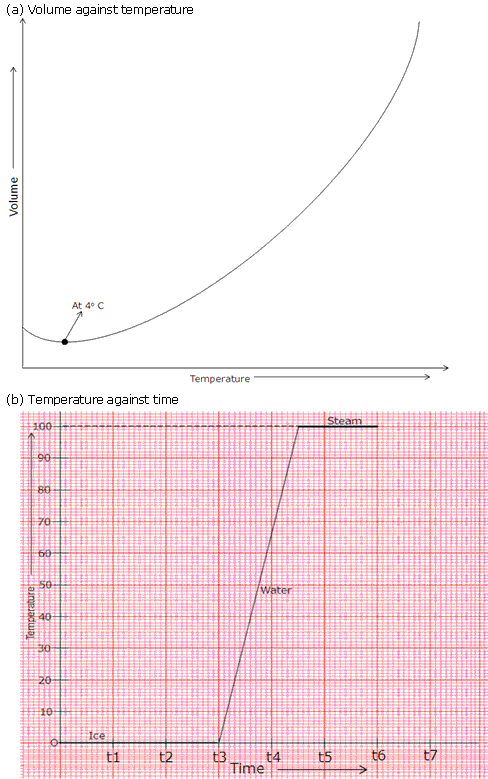
Question 3
Heat is supplied at a constant rate to 200 g of ice at 0oC until finally all the ice is converted into steam at 100oC. If the specific latent heat of ice is 3.3 x 105 J/kg and that of steam is 2.3 x 106 J/kg and the Sp. heat capacity of water is 4200 J/kg, draw rough graphs for the following to illustrate the changes which take place as the solid ice is converted into water and finally into steam, the pressure remaining constant all the time.
(a) Volume against temperature,
(b) Temperature against time.
(a) Volume against temperature,
(b) Temperature against time.
Solution 3
Question 4
Give scientific reasons for the following:
(a) It is much easier to skate on rough ice than on glass.
(b) Sand mixed with salt is often spread over the icy roads in winter.
(c) A steam burn is usually worse than a hot water burn.
(d) Bottled drinks are cooled more effectively when surrounded by lumps of ice than by cold water at 0oC.
(a) It is much easier to skate on rough ice than on glass.
(b) Sand mixed with salt is often spread over the icy roads in winter.
(c) A steam burn is usually worse than a hot water burn.
(d) Bottled drinks are cooled more effectively when surrounded by lumps of ice than by cold water at 0oC.
Solution 4
(a) Ice melts under pressure. So, when the steel blades of the skates pressed on the ice, the ice melts. The water formed makes the skates slide easily over the ice, reducing friction. So, when we are skating on ice, we are skating on a thin film of water, which acts like lubricating oil. Nothing such happens in case of glass.
(b) Sand improves the friction between car tyres and the road, so cars don't skid on icy surfaces. Salt is spread so as to decrease the melting point of ice. Ice on the roads melt, making the roads less slippery.
(c) Steam burn is worse than a hot water burn because 1 g of steam gives out 540 calories of additional heat.
(d) Lumps of ice cool better than cold water because each gram of ice requires additional 80 calories of heat to get converted into water. Hence, cooling capacity of lumps of ice is more than cold water.
(b) Sand improves the friction between car tyres and the road, so cars don't skid on icy surfaces. Salt is spread so as to decrease the melting point of ice. Ice on the roads melt, making the roads less slippery.
(c) Steam burn is worse than a hot water burn because 1 g of steam gives out 540 calories of additional heat.
(d) Lumps of ice cool better than cold water because each gram of ice requires additional 80 calories of heat to get converted into water. Hence, cooling capacity of lumps of ice is more than cold water.
Question 5
A bucket contains 10 liters of water at 80oC. Cold water at 25oC is run from a tap into the bucket of hot water for 20 seconds and the temp. Of the water in the bucket falls to 50oC.
(a) Calculate the rate at which cold water came out of the tap.
(b) State an assumption made in the above calculation.
(a) Calculate the rate at which cold water came out of the tap.
(b) State an assumption made in the above calculation.
Solution 5

Question 6
650 J of heat is required to raise the temp. of 0.25 kg of lead from 15oC to 35oC. Calculate the Sp. heat capacity of lead.
Solution 6
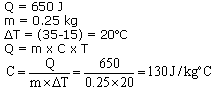
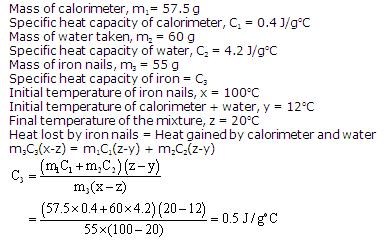
Question 7
A copper calorimeter weighing 57.5 g contains 60 g of water at 12oC. 55 g of iron nails at 100oC are dropped into the calorimeter ands stirred rapidly. The final temperature attained by the calorimeter and its contents is 20oC. Calculate the specific heat of iron. (Sp. heat of copper = 0.4 J/goC, Sp. heat of water = 4.2 J/goC).
Solution 7
Question 8
1 kg of molten lead at its melting point of 327oC is dropped into 1 kg of water at 20oC. Assuming no loss of heat, calculate the final temp. of water. (Sp. heat of lead = 130 J/kgoC, latent heat of lead = 27000 J/kg and Sp. heat of water = 4200 J/kgoC).
Solution 8
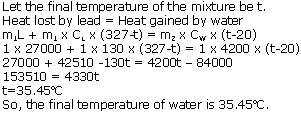
Question 9
Some heat is given to 120 g of water and its temp. rises by 10 K. When the same amount of heat is given to 60 g of oil, its temp. rises by 40 K. The Sp. heat of water is 4200 J/kgK. Calculate:
(a) The amount of heat in joules given to water,
(b) The specific heat capacity of the oil.
(a) The amount of heat in joules given to water,
(b) The specific heat capacity of the oil.
Solution 9
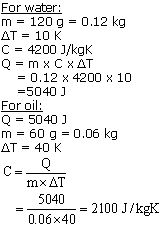
Question 10
A block of lead of mass 250 g, at 27oC was heated in a furnace till it completely melted. Find the quantity of heat required
(a) To bring the lead block upto its melting point,
(b) To completely melt the block at its melting point (Melting point of lead is 3270C, Sp. heat cap. of lead is 0.13 J/gk and latent heat of fusion of lead is 26 J/g)
(a) To bring the lead block upto its melting point,
(b) To completely melt the block at its melting point (Melting point of lead is 3270C, Sp. heat cap. of lead is 0.13 J/gk and latent heat of fusion of lead is 26 J/g)
Solution 10
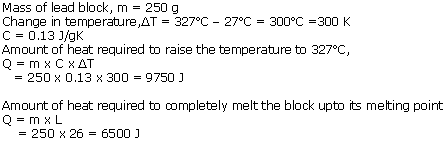
Question 11
100 g of ice at -10oC is heated. It is converted into steam. Calculate the quantity of heat which it has consumed. (Sp. heat of ice = 2100J/kgoC, sp. heat of water= 4200 J/kgK, sp. heat of water = 42000 J/kgK, sp. latent heat of ice = 2260000 J/kg).
Solution 11
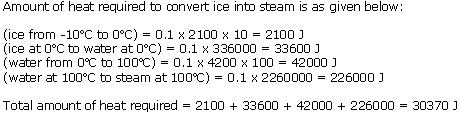
Question 12
A refrigerator converts 100 g of water at 20oC to ice at -10oC in 73.5 minutes. Calculate the average rate of heat extraction from water in watt. (Sp. heat of ice= 2100 J/kgK, sp. heat of water = 4200 J/kgK, Sp. latent heat of ice = 336000 J/kg.)
Solution 12
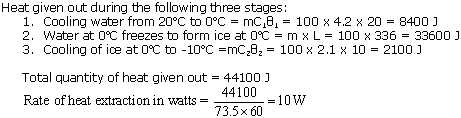
Question 13
A molten metal weighing 150 g is kept at its melting point 800oC. When it is allowed to solidify at the same temp, it gives out 75000 J of heat. What is the specific heat of the metal, if its specific heat capacity is 200 J/kgK? How much additional heat will it give out in cooling to -50oC?
Solution 13
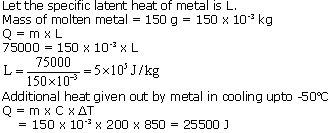
Question 14
40 g of ice at -16oC is dropped into water at 0oC, when 4 g of water freezes into ice. If specific heat capacity of ice is 2100 J/kgoC, what will be the latent heat of fusion of ice?
Solution 14
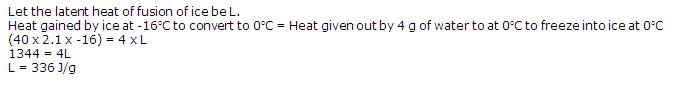
Question 15
Calculate the time taken by an immersion heater, which supplies energy at the rate of 7000 J/minute to raise temp. of 5 kg water from 22oC to 47oC.
Solution 15
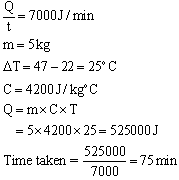
Question 16
In an experiment. 17 g of ice is used to bring down the temp. of 40 g of water from 34oC to its freezing point. The sp. heat capacity of water is 4.25 J/goC. Calculate sp. latent heat of ice.
Solution 16

Question 17
What mass of ice at 0oC will be required to cool 0.9 kg of water from 35oC to 0oC? Assume all the ice used melts. The specific heat capacity of water is 4.2 x 103 J/kgoC and specific latent heat of fusion of ice is 336 x 103 J/kg.
Solution 17
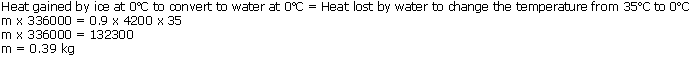
Chapter 5 - Heat - Exercises and MCQ Exercise 248
Question 1
State, with reason, which of the two, boiling water or steam both at 100oC will produce more severe burns.
Solution 1
Steam at 100oC will produce more severe burns because every gram of steam gives out 2260 J of heat energy while condensing. This much amount of heat is additional to the heat contained in one gram of boiling water.
Question 2
Ice cream appears colder to the mouth than water at 0oC. Give a reason.
Solution 2
Ice cream appears colder to mouth than water at 0oC because it can extract approximately 80 cal/g (latent heat of fusion of ice)more heat from as compared to water at 0 oC.
Question 3
Why do bottled soft drinks get cooled, more quickly, by the ice cubes than by the iced water?
Solution 3
Although both ice cubes and iced water are at 0oC but ice cubes cool more quickly because each gram of ice requires additional 80 calories of heat to get converted into water at the same temperature, i.e., at 0oC. Hence, the cooling capacity of ice cubes is more than that of iced water.
Question 4
If 10125 J of heat energy boils off 4.5 g of water at 100oC to steam at 100oC, find the specific latent heat of steam.
Solution 4

Question 5
Fill in the following blanks using suitable word:
(i) SI unit of heat is .........
(ii) 1 cal = .......... J
(iii) Whenever mechanical work is done, ............. Is produced.
(iv) Two bodies in contact are said to be in thermal equilibrium, if they have same ...........
(v) The normal temperature of a human body is .............
(vi) SI unit of specific heat is ..........
(vii) The amount of heat required to change the state of a physical substance without any change of temperature is called .......... of the substance.
(viii) Ice at o0C is colder than ...........at o0C.
(ix) Steam at 100oC is hotter than water at ............
(x) Evaporation causes ............
(i) SI unit of heat is .........
(ii) 1 cal = .......... J
(iii) Whenever mechanical work is done, ............. Is produced.
(iv) Two bodies in contact are said to be in thermal equilibrium, if they have same ...........
(v) The normal temperature of a human body is .............
(vi) SI unit of specific heat is ..........
(vii) The amount of heat required to change the state of a physical substance without any change of temperature is called .......... of the substance.
(viii) Ice at o0C is colder than ...........at o0C.
(ix) Steam at 100oC is hotter than water at ............
(x) Evaporation causes ............
Solution 5

Question 6
Which has more heat: 1 g of ice at 0oC or 1 g of water at 0oC? Give reason.
Solution 6
1 gram of ice at 0oC requires 80 calories of heat to get converted into 1 gram of water at 0oC. So, water has more heat.
Question 7
Which contains more heat: 1 G water at 100oC or 1 g steam at 100oC? Give reason.
Solution 7
1 gram of water at 100oC requires 540 calories of heat to get converted into 1 gram of steam at 100oC. So, steam has more heat.
Question 8
Which requires more heat: 1 g ice at 0oC or 1 g water at 0oC to raise its temperature to 10oC? Explain your answer.
Solution 8
1 gram of ice at 0oC requires additional 80 calories of heat to get converted into water at 0oC. Then, heat is provided to raise the temperature to 10oC. Therefore, ice requires more heat than water and the additional heat is known as 'Latent heat of fusion of ice'.
Question 9
Write down the approximate temperature at which the water boils in a pressure cooker.
Solution 9
Pressure cooker increases the pressure and hence the boiling point increases. So, the boiling point becomes greater than 100oC.

0 comments:
Post a Comment