Chapter 5.2 - Change of State and Latent Heat Exercise 241
Question 1
Define specific heat. Is it same as heat capacity?
Solution 1
Specific heat is the amount of heat required to raise the temperature of 1 kg of the substance through 1oC.
It is not same as heat capacity.
It is not same as heat capacity.
Question 2
Name the physical quantity which has its unit as J/0C.
Solution 2
Heat capacity has units J/oC.
Question 3
What is the value of specific heat of water?
Solution 3
Specific heat of water is 4200 J kg-1K-1.
Question 4
The substances like water which have ........... Heat capacity warm up more slowly than substances like iron which have .......... heat capacity.
Solution 4
The substances like water which have high heat capacity warm up more slowly than substances like iron which have low heat capacity.
Question 5
What do you mean by latent heat?
Solution 5
Latent heat is the quantity of heat absorbed or released by a substance undergoing a change of state, such as ice changing to water or water to steam, at constant temperature.
Question 6
Write the SI unit of latent heat.
Solution 6
SI unit of latent heat is J/kg.
Question 7
What do you mean by the statement?
'The specific latent heat capacity of fusion of ice is 336 J per g'?
'The specific latent heat capacity of fusion of ice is 336 J per g'?
Solution 7
It means that 1 g of ice at 0oC absorbs 336 J of heat energy to convert into water at 0oC.
Question 8
When a liquid is solidified, it many either expand or contract. As water freezes to form ice it ........... And .............. in volume by ............. per cent.
Solution 8
When a liquid is solidified, it may either expand or contract. As water freezes to form ice it expands and increases in volume by 10 per cent.
Question 9
What is the effect of impurities on the melting point of ice?
Solution 9
The melting point of ice decreases on addition of impurities in it.
Question 10
What is the effect of pressure on the melting point of ice?
Solution 10
The melting point of substances which contract on melting (like ice) decreases by the increase in pressure.
Question 11
What do you mean by regelation?
Solution 11
Regelation is the phenomenon of melting under pressure and freezing again when the pressure is reduced.
Question 12
Write the SI unit of specific heat.
Solution 12
SI unit of specific heat is J kg-1K-1.
Question 13
What do you understand by the latent heat of vaporization?
Solution 13
The amount of heat required to change a liquid at its boiling point to vapour at the same temperature is called its latent heat of vaporization.
Question 14
What is the effect of pressure on the boiling point of water?
Solution 14
The boiling point of a liquid increases with the increase in pressure and decreases with the decrease in pressure.
Question 15
What is the latent heat of fusion of ice?
Solution 15
The latent heat of fusion of ice is the amount of heat energy required to change ice at 0oC into water at the same temperature.
Question 16
What is the latent heat of vaporization of steam?
Solution 16
The latent heat of vaporization of steam is the amount of heat energy required to change water at 100oC to steam at the same temperature.
Question 17
The physical quantity which does not change during change of state is ............. of the body.
Solution 17
The physical quantity which does not change during change of state is temperature of the body.
Question 18
Why does ice appear colder than water at 00C?
Solution 18
1 kg of ice at 0oC absorbs 336000 J of heat energy to convert into water at 0oC. Therefore,1 kg of water at 0oC has 336000 J heat energy more than 1 kg ice at 0oC. So, ice appears colder than water.
Question 19
Why does cause more severe burns than water at 1000C?
Solution 19
Steam causes more severe burns than water at 100oC because every gram of steam gives out 2260 J of heat energy while condensing. This much quantity of heat is additional to the heat contained in 1 g of boiling water.
Question 20
What is the unit of heat capacity in CGS system?
Solution 20
The unit of heat capacity in CGS system is calorie/oC.
Question 21
1 cal g-1 0C-1 = ........... J kg-1 0C-1.
Solution 21
1 cal g-1 oC-1 = 239 J kg-1 oC-1
Question 22
The specific heat of body is 0.2 cal g-1 0C-1. What is the meaning of this statement?
Solution 22
This means that 0.2 cal g-1 oC-1 of heat is required to raise the temperature of 1g of the body by 1oC.
Question 23
Name the physical quantity which has its unit as j/(kg)/(K).
Solution 23
Specific Heat.
Question 24
The specific heat of a substance of mass 100 g is 0.04 cal g-1 0C-1. What is its heat capacity?
Solution 24
m = 100 g
C = 0.04 cal g-1 oC-1
Heat capacity = m x C = 4 cal oC-1
C = 0.04 cal g-1 oC-1
Heat capacity = m x C = 4 cal oC-1
Question 25
Write down the relation between specific heat and heat capacity.
Solution 25
Heat capacity = mass x specific heat capacity
Question 26
Does specific heat depend upon the mass of a substance?
Solution 26
No, specific heat does not depend on mass of a substance. It is constant for a substance.
Question 27
How much is the specific heat of water in SI units?
Solution 27
Specific heat of water in SI units is 4200 J kg-1 K-1.
Question 28
Name the substance that has the maximum value of specific heat.
Solution 28
Ammonia has the maximum value of specific heat.
Question 29
State the factors on which heat gained or lost by a substance depends.
Solution 29
Heat gained or lost by a substance depends on the mass of the substance and the nature of the substance.
Question 30
Why is the ocean known as storehouse of heat energy?
Solution 30
Oceans cover more than 70% of Earth's surface, making them the world's largest solar collectors. The sun's heat warms the surface water a lot more than the deep ocean water, and this temperature difference creates heat energy. Thus, oceans are known as storehouse of heat energy. Just a small portion of the heat trapped in the ocean could power the world.
Question 31
Why do we use water as a cooling agent in the radiators of automobiles?
Solution 31
Water has a high specific heat capacity. So, water extracts much heat without much rise in temperature. By allowing water to flow in radiator pipes of the vehicles, heat energy form such parts is removed. Hence, it is used as a cooling agent in the radiators of automobiles.
Question 32
State the principle of calorimetry.
Solution 32
Principle of Calorimetry:
When a hot body is mixed or kept in contact with a cold body, there is a transfer of heat from hot body to cold body such that
Total heat gained by colder body = Total heat lost by the hot body,
if there is no loss of heat to the surroundings.
When a hot body is mixed or kept in contact with a cold body, there is a transfer of heat from hot body to cold body such that
Total heat gained by colder body = Total heat lost by the hot body,
if there is no loss of heat to the surroundings.
Question 33
What do you mean by thermal capacity?
Solution 33
Thermal capacity of a body is the quantity of heat required to raise its temperature by 1oC.
Question 34
The product of mass and specific heat is known as ..........
Solution 34
The product of mass and specific heat is known as heat capacity.
Question 35
Does the specific heat depend upon the temperature?
Solution 35
No, specific heat does not depend on temperature. It is constant for a substance.
Question 36
Why does a copper rod become warmer than an aluminium rod of the same mass, when put in the sun for the same length of time?
Solution 36
Copper road becomes warmer than an aluminium rod of the same mass because copper has lower heat capacity than aluminium.
Question 37
The amount of heat required to raise the temperature of a body by 10C is called .........
Solution 37
The amount of heat required to raise the temperature of a body by 1oC is called Heat capacity.
Chapter 5.2 - Change of State and Latent Heat Exercise 242
Question 1
What is meant by change of state?
Solution 1
A change of state is a change in the object from a solid to liquid or from a solid to gas or from liquid to solid. Ice changing into water is an example of a change in state.
Question 2
State whether heat energy is absorbed or released during melting of ice.
Solution 2
Heat energy is absorbed during melting of ice.
Question 3
State whether heat energy is absorbed or released during freezing of ice.
Solution 3
Heat energy is released during freezing of ice.
Question 4
Temperature ............ When ice melts at 00C.
Solution 4
Temperature remains constant when ice melts at 0oC.
Question 5
Why does the temperature of a substance remain constant during the process of melting?
Solution 5
The molecules in a solid are held by strong intermolecular bonds. For the solid to melt, these bonds have to be broken. Since energy is needed to break the intermolecular bonds, the thermal energy supplied at the melting point is used to do the work to break the intermolecular bonds between the molecules of the solid. Once the intermolecular bonds are broken, the molecules can then move out of their fixed positions. Hence it can then be said that the solid has melted, which is the change of state from solid to liquid. This explains why temperature remains constant during the melting phases.
Question 6
Why does the temperature of a substance remain constant during the process of boiling?
Solution 6
Whenever a substance goes through a phase change (like boiling), the energy goes into breaking up the interactions between molecules, and so the temperature stays constant until all the interactions are broken. Once whole of the substance has boiled, then any added heat will act to raise them temperature again.
Question 7
Atmosphere is usually warm during snowfall. Why?
Solution 7
Atmosphere is usually warm during snowfall because each kilogram of ice on melting absorbs 336000 J of heat from atmosphere.
Question 8
Find the amount of heat required to convert 2 g of ice at 00C into water at 00C. Latent heat of fusion of ice= 336 Jg-1.
Solution 8
Latent heat of fusion of ice, L=336 J/g
Mass of ice, m=2g
Amount of heat required convert 2g of ice at 0oC into water at 0oC = m x L
= 2 x 336 = 672 J
Mass of ice, m=2g
Amount of heat required convert 2g of ice at 0oC into water at 0oC = m x L
= 2 x 336 = 672 J
Question 9
Find the amount of heat required to convert 100 g of water at 1000C into steam at 1000C. Latent heat of vapourisation of water is 540 calg-1.
Solution 9
Latent heat of vapourisation of water, L=540 cal/g
Mass of water, m=100g
Amount of heat required convert 100g of water at 100oC into steam at 100oC = m x L
= 100 x 540 = 5.4 kJ
Mass of water, m=100g
Amount of heat required convert 100g of water at 100oC into steam at 100oC = m x L
= 100 x 540 = 5.4 kJ
Question 10
It is much easier to skate on rough ice than on glass. Give reasons.
Solution 10
Ice melts under pressure. So, when the steel blades of the skates pressed on the ice, the ice melts. The water formed makes the skates slide easily over the ice, reducing friction. So, when we are skating on ice, we are skating on a thin film of water, which acts like lubricating oil. Nothing such happens in case of glass.
Question 11
Bottled drinks are cooled more effectively when surrounded by lamps of ice than by cold water at 00C. Why?
Solution 11
Bottled drinks are cooled more effectively when surrounded by lumps of ice than by cold water at 0oC because ice appears colder than water at 0oC.
Question 12
A substance is heated at a constant rate from a low temperature to a high temperature. A graph of temperature against time is shown in the figure. Which part or parts of the graph correspond(s) to the substance existing in two states?
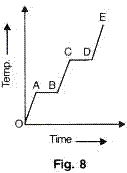

Solution 12
Parts AB and CD correspond to the substance existing in two states.
Question 13
A solid is heated at a constant rate in an insulated container till half of it vaporizes. Which of the following graphs show the temperature changes during this time?
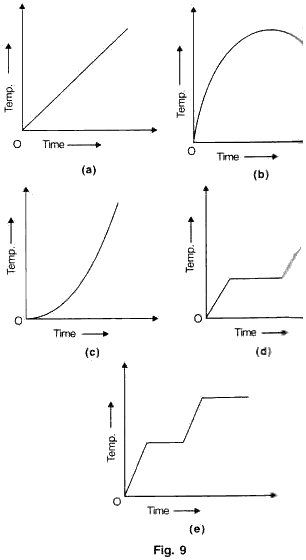

Solution 13
(e) The first time when temperature is constant represents change of state from solid to liquid and the second time temperature is constant represents change of state from liquid to vapour.
Question 14
The graph given below represents a cooling curve fore a substance being cooled from a higher temperature to a lower temperature.
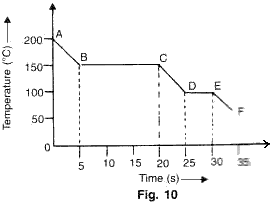
(a)What is the boiling point of the substance?
(b)What happens in the region DE?
(c)What is the melting point of the substance?

(a)What is the boiling point of the substance?
(b)What happens in the region DE?
(c)What is the melting point of the substance?
Solution 14
(a)Boiling point of substance is 150oC (because the part BC represents condensation where the vapour changes into liquid without the change in temperature.
(b)DE represents freezing of the substance where the liquid changes into solid at a constant temperature of 100oC.
(c)Melting point is the temperature of the region DE where liquid changes into solid i.e., 100oC.
(b)DE represents freezing of the substance where the liquid changes into solid at a constant temperature of 100oC.
(c)Melting point is the temperature of the region DE where liquid changes into solid i.e., 100oC.
Chapter 5.2 - Change of State and Latent Heat Exercise 243
Question 1
A slab of ice at -500C is constantly heated till the steam attains a temperature of 1500C. Draw a graph showing the change of temperature with time. Label the various parts of graph properly.
Solution 1
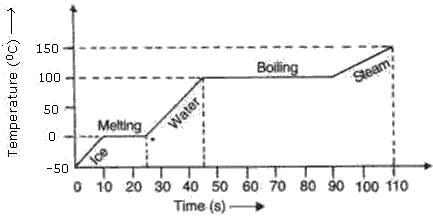
Question 2
A piece of ice is heated at a constant rate. The variation in temperature with time of heating is shown in the graph (see fig. 12).
(i) What is represented by AB?
(ii) What does CD represent?
(iii) What conclusion can you draw regarding the nature of ice from the graph below?
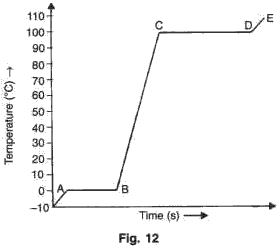
(i) What is represented by AB?
(ii) What does CD represent?
(iii) What conclusion can you draw regarding the nature of ice from the graph below?

Solution 2
i.AB represents the change of state from solid to liquid i.e., AB represents melting of ice at 0oC.
ii.CD represents the change of state from liquid to vapour i.e., CD represents boiling of water at 100oC.
iii.The ice initially is in solid state at -10oC. On heating, its temperature rises to 0oC. It then takes some heat at 0oC to melt in water at 0oC which is its latent heat.
ii.CD represents the change of state from liquid to vapour i.e., CD represents boiling of water at 100oC.
iii.The ice initially is in solid state at -10oC. On heating, its temperature rises to 0oC. It then takes some heat at 0oC to melt in water at 0oC which is its latent heat.

0 comments:
Post a Comment