Chapter 5 - Mole Concept And Stoichiometry Exercise 115
Question 1
State:
(a) Gay-Lussac's law of combining volumes
(b) Avogadro's law
(a) Gay-Lussac's law of combining volumes
(b) Avogadro's law
Solution 1
(a) Gay-Lussac's law: It states that 'when gases react, they do so in volumes which bear a simple ratio to one another, and also to the volume of the gaseous product, provided all the volumes are measured at the same temperature and pressure'.
(b) Avogadro's law : It states that 'Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules'.
(b) Avogadro's law : It states that 'Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules'.
Question 2
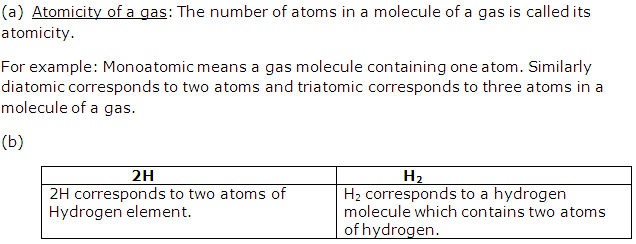
(a) Define atomicity of a gas.
(b) Differentiate between 2H and H2.
(b) Differentiate between 2H and H2.
Solution 2
Question 3
When stating the volume of a gas, the pressure and temperature should also be given. Why?
Solution 3
When stating the volume of a gas, the pressure and temperature should also be given because the volume of a gas is highly susceptible to slight change in pressure and temperature of the gas.
Question 4
(a) The relative atomic mass of Cl atom is 35.5 a.m.u. Explain this statement.
(b) What is the value of Avogadro's number?
(c) What is the value of molar volume of a gas at STP?
(b) What is the value of Avogadro's number?
(c) What is the value of molar volume of a gas at STP?
Solution 4
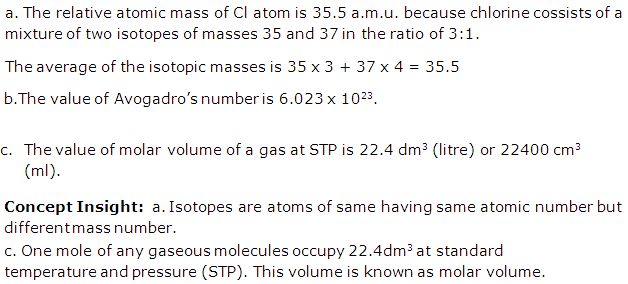
Question 5
Define the terms:
(a) Vapour density.
(b) Molar volume.
(c) Relative atomic mass.
(d) Avogadro's number.
(e) Relative molecular mass.
(a) Vapour density.
(b) Molar volume.
(c) Relative atomic mass.
(d) Avogadro's number.
(e) Relative molecular mass.
Solution 5

Question 6
(a) What are the main applications of Avogadro's law?
(b) How does Avogadro's law explain Gay - lussac's law of combining volumes?
(b) How does Avogadro's law explain Gay - lussac's law of combining volumes?
Solution 6

Question 7
Explain the terms:
(a) Gram atom
(b) Gram mole
(c) Mole
(a) Gram atom
(b) Gram mole
(c) Mole
Solution 7
(a) Gram atom: "The quantity of the element which weighs equal to its gram atomic mass is called one gram atom of that element".
For example: The gram atomic mass of hydrogen is 1g. So, 1g of hydrogen is 1 gram atom of hydrogen.
(b) Gram mole: "A sample of substance with its mass equal to its gram molecular mass is called one gram molecule of this substance or one gram mole".
For example: Gram molecular mass of oxygen is 32 g. So One gram mole of oxygen is 32g.
For example: The gram atomic mass of hydrogen is 1g. So, 1g of hydrogen is 1 gram atom of hydrogen.
(b) Gram mole: "A sample of substance with its mass equal to its gram molecular mass is called one gram molecule of this substance or one gram mole".
For example: Gram molecular mass of oxygen is 32 g. So One gram mole of oxygen is 32g.
Question 8
Calculate the relative molecular masses of (use K = 39, Cl = 35.5, O = 16, C = 12, H = 1, Na = 23, N = 14, S= 32)
(a) Potassium chlorate
(b) Sodium acetate
(c) Chloroform
(d) Ammonium sulphate
(a) Potassium chlorate
(b) Sodium acetate
(c) Chloroform
(d) Ammonium sulphate
Solution 8

Question 9
Explain the terms empirical formula and molecular formula.
Solution 9
Empirical formula:"Empirical formula of a compound is the formula which gives the number of atoms of different elements present in one molecule of the compound, in the simplest numerical ratio".
Molecular formula: "Molecular formula of a compound denotes the actual number of atoms of different elements present in one molecule of the compound".
Molecular formula: "Molecular formula of a compound denotes the actual number of atoms of different elements present in one molecule of the compound".
Question 10
Give the empirical formula of:
(a) C6H6
(b) C6H12O6
(c) C2H2
(d) CH3COOH
(a) C6H6
(b) C6H12O6
(c) C2H2
(d) CH3COOH
Solution 10
(a) The empirical formula of C6H6 is: CH
(b) The empirical formula of C6H12O6 is: CH2O.
(c) The empirical formula of C2H2 is: CH
(d) The empirical formula of CH3COOH is: CH2O.
(b) The empirical formula of C6H12O6 is: CH2O.
(c) The empirical formula of C2H2 is: CH
(d) The empirical formula of CH3COOH is: CH2O.
Question 11
Give three pieces of information conveyed by the formula H2O.
Solution 11
Three pieces of information conveyed by the formula H2O is that:
It shows that there are 2hydrogen atoms and 1oxygen atoms present in H2O.
The hydrogen and oxygen atoms are present in simplest whole number ratio of 2:1.
It represents one molecule of compound water.
It shows that there are 2hydrogen atoms and 1oxygen atoms present in H2O.
The hydrogen and oxygen atoms are present in simplest whole number ratio of 2:1.
It represents one molecule of compound water.
Question 12
What do you understand by the term mole? How many elementary units are in one mole of a substance?
Solution 12
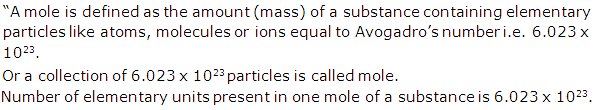
Question 13
Fill in the blanks:
(a) Molecular weight of a gas is twice its ______.
(b) Mass of 22.4 litre of a gas at STP is ______.
(c) One gram atom of an element contains ______ atoms.
(d) One a.m.u. is the mass of ______ atom of C12.
(e) Avogadro's number is equal to ______.
(a) Molecular weight of a gas is twice its ______.
(b) Mass of 22.4 litre of a gas at STP is ______.
(c) One gram atom of an element contains ______ atoms.
(d) One a.m.u. is the mass of ______ atom of C12.
(e) Avogadro's number is equal to ______.
Solution 13
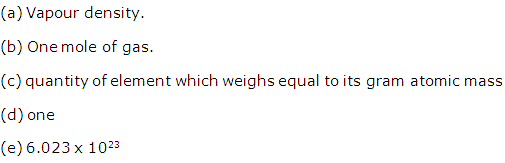
Question 14
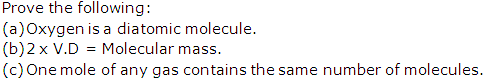
Solution 14
Question 15
Give examples of compounds whose:
(a) Empirical formula is the same as the molecular formula.
(b) Empirical formula is different from the molecular formula.
(a) Empirical formula is the same as the molecular formula.
(b) Empirical formula is different from the molecular formula.
Solution 15
(a) Na2SO4.10H2O.
(b) C6H12O6.
(b) C6H12O6.
Chapter 5 - Mole Concept And Stoichiometry Exercise 116
Question 1
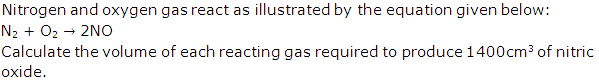
Solution 1

Question 2
Solution 2
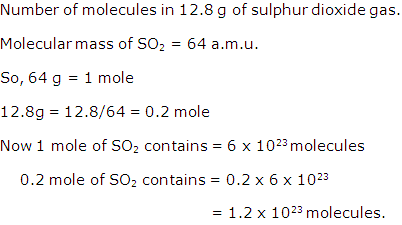
Question 3
Solution 3
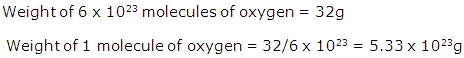
Question 4
Calculate the percentage composition of oxygen in lead nitrate [Pb(NO3)2]. [Pb = 207, N= 14, O = 16]
Solution 4
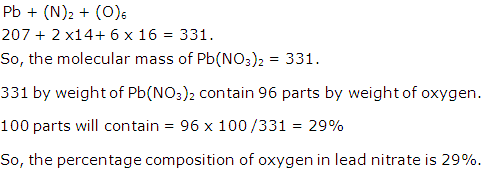
Question 5
The usefulness of a fertilizer depends upon percentage of nitrogen present in it. Find which of the following is a better fertilizer:
(a) Ammonium nitrate [NH4NO3]
(b) Ammonium phosphate [(NH4)3PO4] (N=14,H=1,O=16,P=31)
(a) Ammonium nitrate [NH4NO3]
(b) Ammonium phosphate [(NH4)3PO4] (N=14,H=1,O=16,P=31)
Solution 5
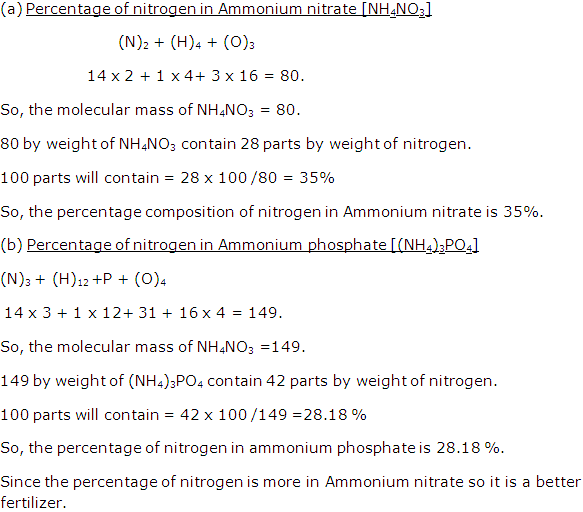
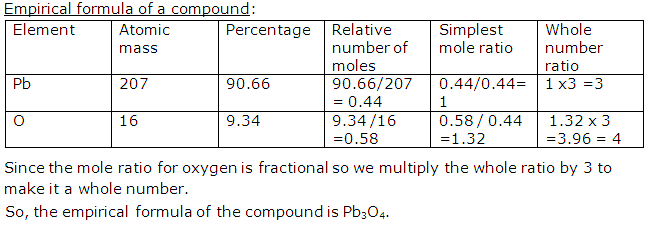
Question 6
A compound of lead has following percentage composition, Pb = 90.66%, O = 9.34%. Calculate empirical formula of a compound. [Pb = 207, O = 16]
Solution 6
Question 7
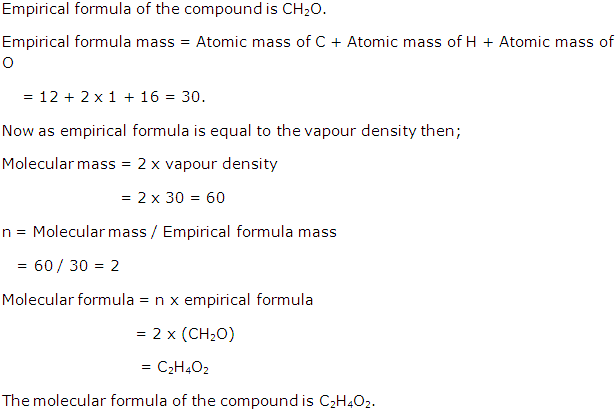
Empirical formula of a compound is CH2O. If its empirical formula is equal to its vapour density, calculate the molecular formula of the compound.
Solution 7
Question 8
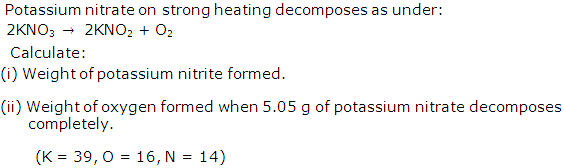
Solution 8
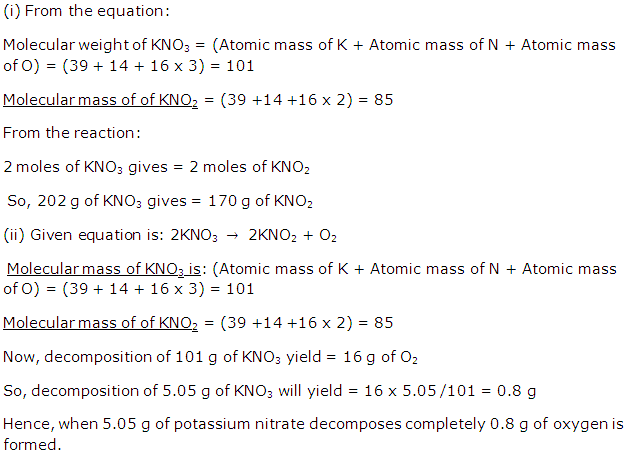
Question 9
(a)What is the volume occupied by the following gases at STP?
(i) 48 g of oxygen.
(ii) 16 g of sulphur dioxide.
(b) Determine the molecular mass of a gas if 5g of it occupy a volume of 4 L at STP.
(i) 48 g of oxygen.
(ii) 16 g of sulphur dioxide.
(b) Determine the molecular mass of a gas if 5g of it occupy a volume of 4 L at STP.
Solution 9

Question 10
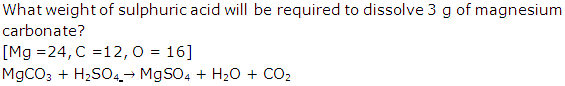
Solution 10
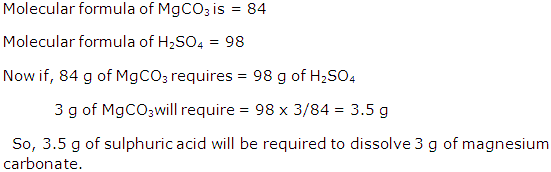
Question 11
Calculate the percentage of water in ferrous sulphate crystals [Fe = 56, S = 32, O =16, H = 1].
Solution 11

Question 12
An organic compound has the following percentage composition: C = 12.76%, H = 2.13%, Br = 85.11%. The vapour density of the compound is 94. Find out its molecular formula.
Solution 12
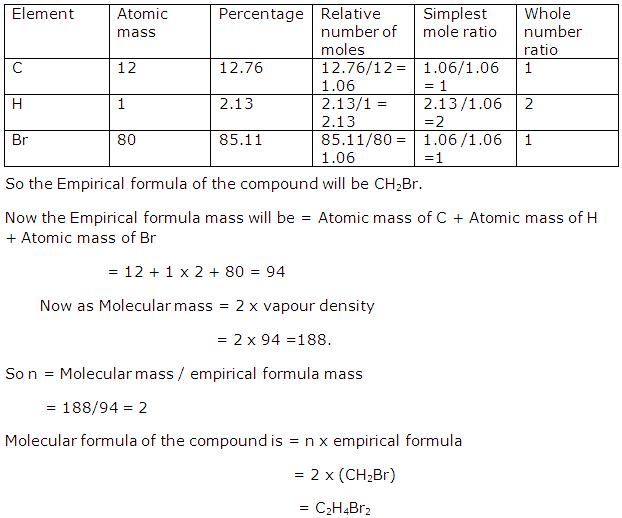
Question 13
Two oxides of a metal (M) have 20.12% and 11.19% oxygen. The formula of the first oxide is MO. Determine the formula of the second oxide.
Solution 13
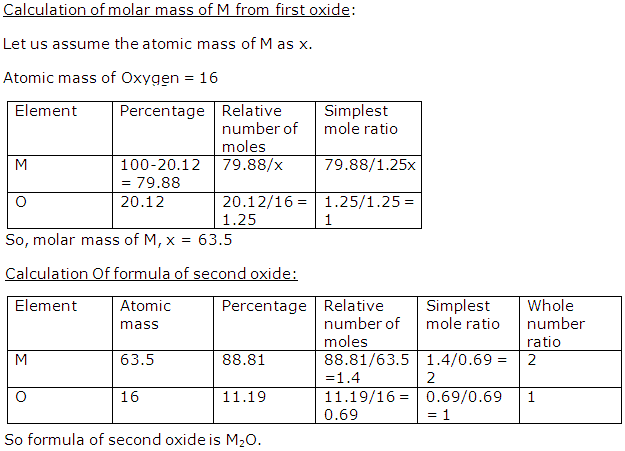
Question 14
Calculate the volume of air at STP, required to convert 300 mL of sulphur dioxide to sulphur trioxide. Air contains 21% of oxygen by volume.
Solution 14

Question 15
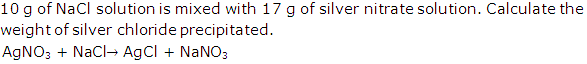
Solution 15
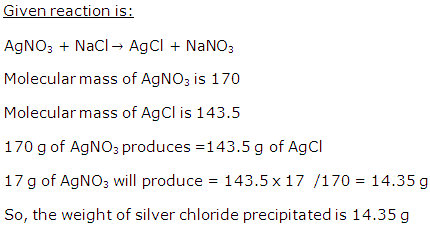
Question 16
A student puts his signature with graphite pencil. If the mass of carbon in the signature is 10-12 g. Calculate the number of carbon atoms in the signature.
Solution 16
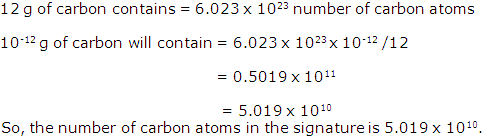
Chapter 5 - Mole Concept And Stoichiometry Exercise 117
Question 1
Under the same conditions of temperature and pressure you collect 2 L of carbon dioxide, 3L of chlorine, 5 L of hydrogen, 4 L of nitrogen and 1 L of sulphur dioxide. In which gas sample will there be :
(a) The greatest number of molecules.
(b) The least number of molecules.
Justify your answer.
(a) The greatest number of molecules.
(b) The least number of molecules.
Justify your answer.
Solution 1

Question 2

Solution 2

Question 3
Find the total percentage of oxygen in magnesium nitrate crystal Mg(NO3)2.6H2O.
[H = 1, N = 14, O = 16, Mg = 24]
[H = 1, N = 14, O = 16, Mg = 24]
Solution 3
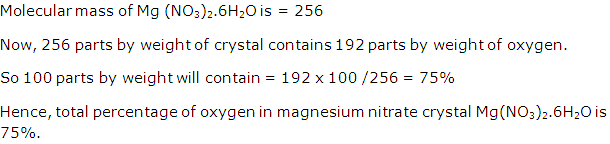
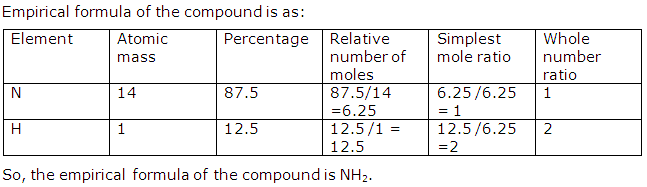
Question 4
A compound contains 87.5% by mass of nitrogen and 12.5% by mass of hydrogen. Determine the empirical formula of this compound.
Solution 4
Question 5
(a) Is it possible to change the temperature and pressure of a fixed mass of a gas without changing its volume? Explain your answer.
(b) Define or explain the meaning of term 'molar volume'.
(b) Define or explain the meaning of term 'molar volume'.
Solution 5

Question 6
What is the mass of nitrogen in 1000 Kg of urea [CO(NH2)2] ?
[H = 1, C= 12, N= 14, O = 16]
[H = 1, C= 12, N= 14, O = 16]
Solution 6
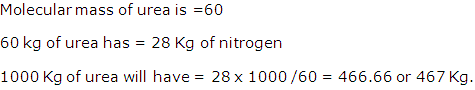
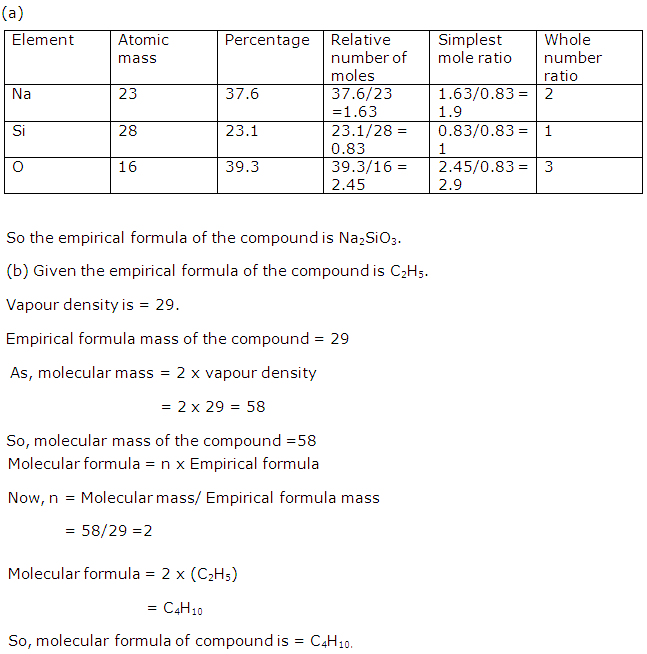
Question 7
(a) Calculate the empirical formula of the compound having 37.6% sodium, 23.1% silicon and 39.3% oxygen.(Answer correct to two decimal places) (O = 16, Na = 23, Si = 28)
(b) The empirical formula of a compound is C2H5. Its vapour density is 29. Determine the relative molecular mass of the compound and hence its molecular formula.
(b) The empirical formula of a compound is C2H5. Its vapour density is 29. Determine the relative molecular mass of the compound and hence its molecular formula.
Solution 7
Question 8
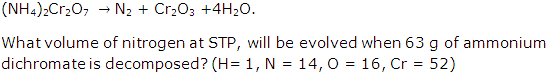
Solution 8
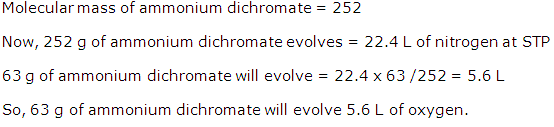
Question 9
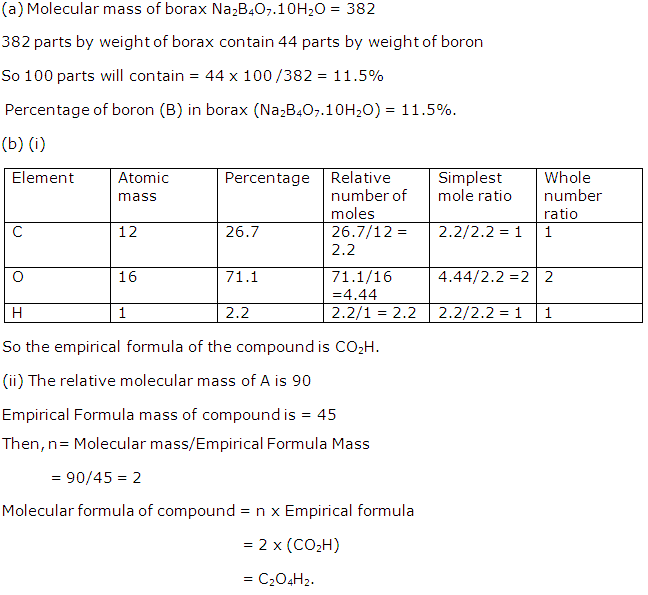
(a) Calculate the percentage of boron (B) in borax (Na2B4O7.10H2O). [H = 1, B = 11, O = 16, Na = 23] , answer correct to 1 decimal place).
(b) (i) The compound A has the following percentage composition by mass:
C =26.7%, O = 71.1%, H = 2.2%. Determine the empirical formula of A.(Answer to one decimal place) (H=1,C=12,O=16)
(ii) If the relative molecular mass of A is 90, what is the molecular formula of A?
(b) (i) The compound A has the following percentage composition by mass:
C =26.7%, O = 71.1%, H = 2.2%. Determine the empirical formula of A.(Answer to one decimal place) (H=1,C=12,O=16)
(ii) If the relative molecular mass of A is 90, what is the molecular formula of A?
Solution 9
Chapter 5 - Mole Concept And Stoichiometry Exercise 118
Question 1
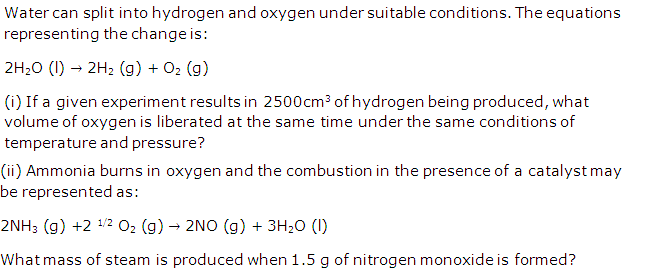
Solution 1

Question 2

Solution 2

Question 3
If a crop of wheat removes 20 Kg of nitrogen per hectare of soil, what mass of the fertilizer calcium nitrate, Ca(NO3)2 would be required to replace nitrogen in 10 hectare field? (N = 14, O = 16, Ca = 40)
Solution 3

Question 4
(a) A vessel contains N molecules of oxygen at a certain temperature and pressure. How many molecules of sulphur dioxide can the vessel accommodate at the same temperature and pressure?
(b) Each of the two flasks contains 2.0 g of gas at the same temperature and pressure. One flask contains oxygen and the other hydrogen.
(i) Which sample contains the greater number of molecules?
(ii) If the hydrogen sample contains N molecules, how many molecules are present in oxygen sample?
(b) Each of the two flasks contains 2.0 g of gas at the same temperature and pressure. One flask contains oxygen and the other hydrogen.
(i) Which sample contains the greater number of molecules?
(ii) If the hydrogen sample contains N molecules, how many molecules are present in oxygen sample?
Solution 4

Question 5
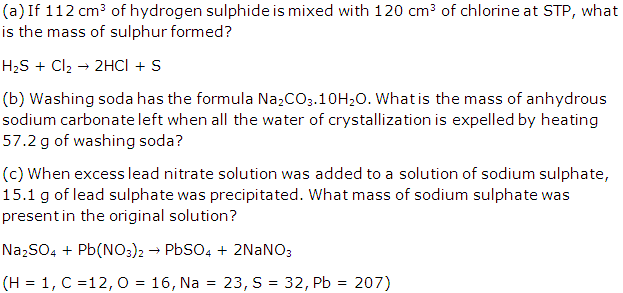
Solution 5

Question 6
Determine the empirical formula of the compound whose composition by mass is 42% nitrogen, 48% oxygen and 9% hydrogen.[N=14,O=16,H=1]
Solution 6
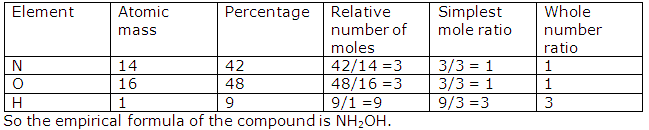
Chapter 5 - Mole Concept And Stoichiometry Exercise 119
Question 1
When gases react their volumes bear a simple ratio to each other, under the same conditions of temperature and pressure. Who proposed this gas law?
Solution 1
Gay - Lussac proposed this law.
Question 2
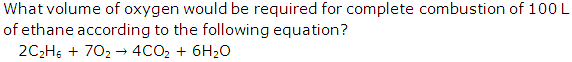
Solution 2
Molecular mass of ethane = 30
According to Gay-Lussac's law:
2 vol. of C2H6 requires= 7 vol. of oxygen
Vol. of C2H6 = 2 vol. = 100 L
Vol. of oxygen required = 7 vol. =350 L
According to Gay-Lussac's law:
2 vol. of C2H6 requires= 7 vol. of oxygen
Vol. of C2H6 = 2 vol. = 100 L
Vol. of oxygen required = 7 vol. =350 L
Question 3
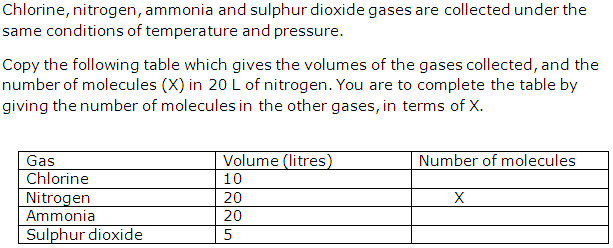
Solution 3
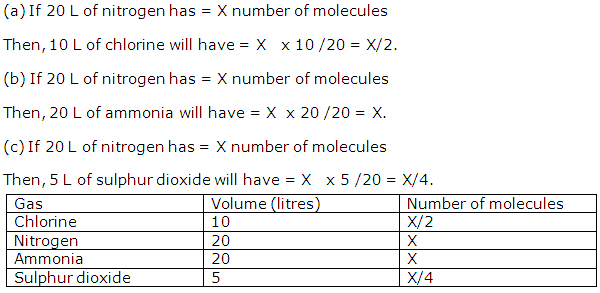
Question 4
Mention the term defined by the following sentence:
The mass of a given volume of gas compared to the mass of an equal volume of hydrogen.
The mass of a given volume of gas compared to the mass of an equal volume of hydrogen.
Solution 4
The term is vapour density.
Question 5
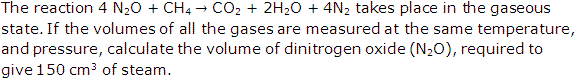
Solution 5
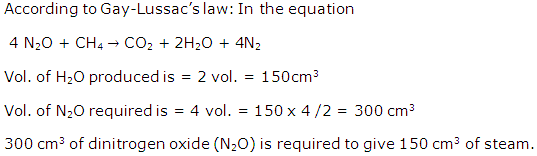
Question 6
Calculate the percentage of phosphorous in the fertilizer superphosphate, Ca(H2PO4)2. [Ca = 40, H =1, P =31, O = 16] (Correct to 1 decimal place)
Solution 6
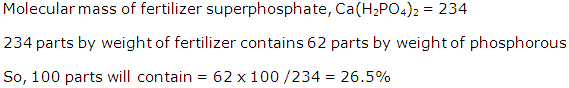
Question 7
A metal M, forms a volatile chloride containing 65.5% Chlorine. If the density of the chloride relative to hydrogen is 162.5, find the molecular formula of the chloride. [M = 56, Cl = 35.5]
Solution 7
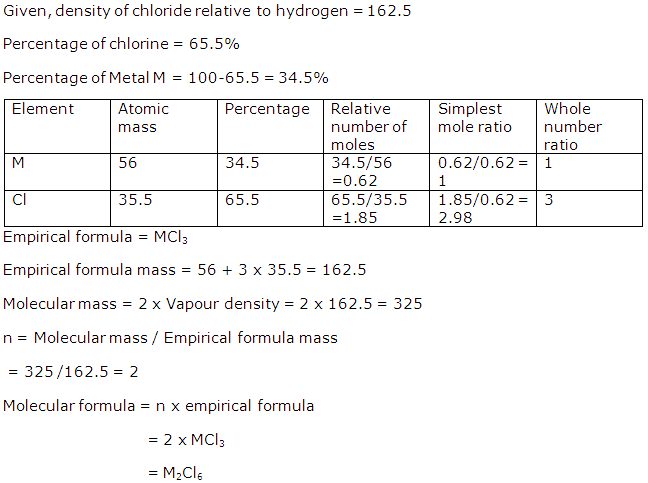
Question 8
Samples of the gases O2, N2, CO2 and CO under the same conditions of temperature and pressure contain the same number of molecules represented by X. The molecules of Oxygen, occupy V litres and have a mass of 8 g. Under the same conditions of temperature and pressure:
(a) What is the volume occupied by:
(i) X molecules of N2,
(ii) 3X molecules of CO? [C=12,N=14,O=16]
(b) What is the mass of CO2 in g?
(c) In answering the above questions, which law have you used?
(a) What is the volume occupied by:
(i) X molecules of N2,
(ii) 3X molecules of CO? [C=12,N=14,O=16]
(b) What is the mass of CO2 in g?
(c) In answering the above questions, which law have you used?
Solution 8
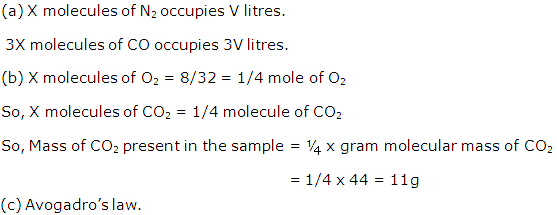
Question 9
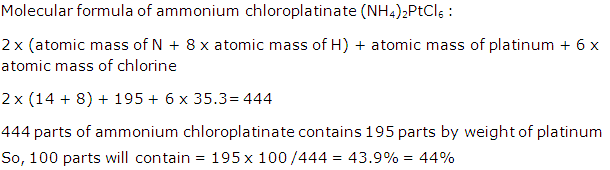
Calculate the percentage of platinum in ammonium chloroplatinate (NH4)2PtCl6. [N = 14, H = 1, Pt = 195, Cl =35.5] (Give your answer correct to the nearest whole number)
Solution 9
Question 10
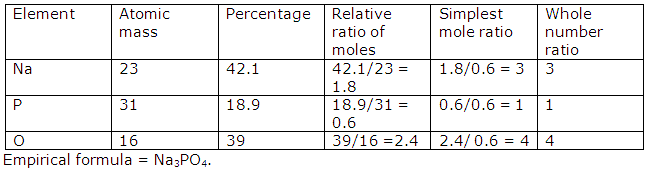
The percentage composition of sodium phosphate, as determined by analysis is: 42.1% Na, 18.9% P, 39% of O. Find the empirical formula of the compound. [H =1, N =14, Na = 23, P = 31, Cl = 35.5, Pt = 195]
Solution 10
Chapter 5 - Mole Concept And Stoichiometry Exercise 120
Question 1
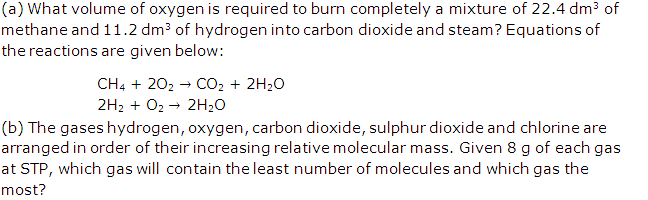
Solution 1

Question 2

Solution 2

Question 3
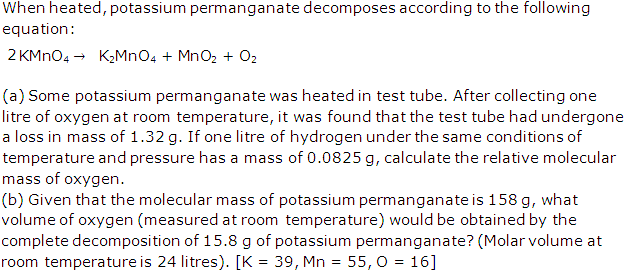
Solution 3
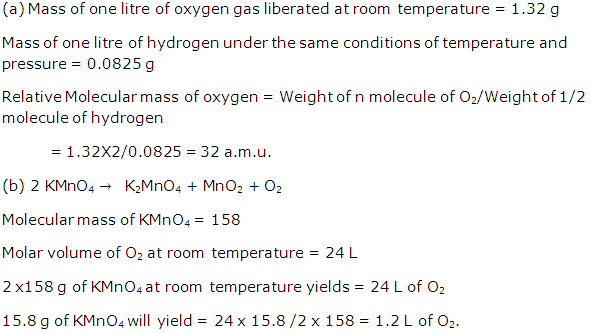
Question 4

Solution 4
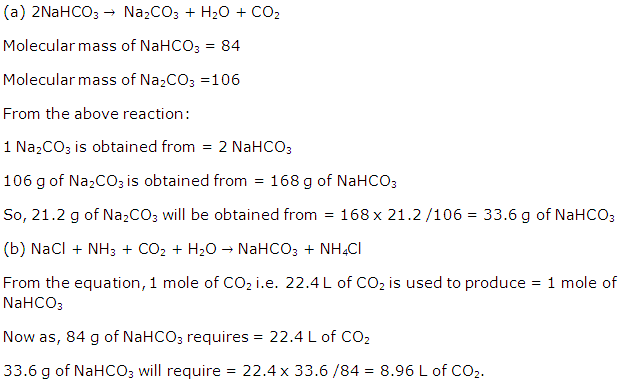
Chapter 5 - Mole Concept And Stoichiometry Exercise 121
Question 1
(a) The volumes of gases A, B, C and D are in the ratio, 1:2:2:4 under the same conditions of temperature and pressure.
(i) Which sample of gas contains the maximum number of molecules?
(ii) If the temperature and the pressure of gas A are kept constant then what will happen to the volume of A when the number of molecules is doubled?
(iii) If this ratio of gas volumes refers to the reactants and products of a reaction, which gas law is being observed?
(iv) If the volume of A is actually 5.6 dm3 at STP, calculate the number of molecules in the actual volume of D at STP.
(v) Using your answer from (iv), state, the mass of D if the gas is dinitrogen oxide (N2O)
(b) Calculate the percentage of nitrogen in aluminium nitride. [Al = 27, N = 14]
(i) Which sample of gas contains the maximum number of molecules?
(ii) If the temperature and the pressure of gas A are kept constant then what will happen to the volume of A when the number of molecules is doubled?
(iii) If this ratio of gas volumes refers to the reactants and products of a reaction, which gas law is being observed?
(iv) If the volume of A is actually 5.6 dm3 at STP, calculate the number of molecules in the actual volume of D at STP.
(v) Using your answer from (iv), state, the mass of D if the gas is dinitrogen oxide (N2O)
(b) Calculate the percentage of nitrogen in aluminium nitride. [Al = 27, N = 14]
Solution 1

Question 2
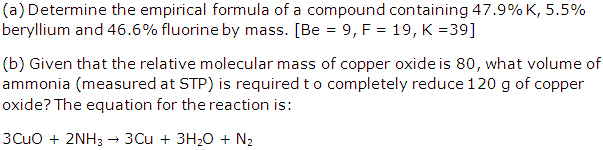
Solution 2
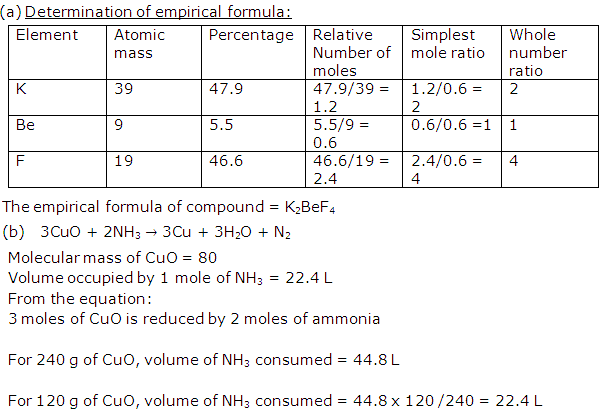
Question 3
(a) Calculate the number of moles and number of molecules present in 1.4 g of ethylene gas. What is the volume occupied by the same amount of ethylene?
(b) What is the vapour density of ethylene? (Avogadro's number = 6x1023; Atomic weight of C = 12, H = 1; Molar volume = 22.4 litres at STP)
(b) What is the vapour density of ethylene? (Avogadro's number = 6x1023; Atomic weight of C = 12, H = 1; Molar volume = 22.4 litres at STP)
Solution 3
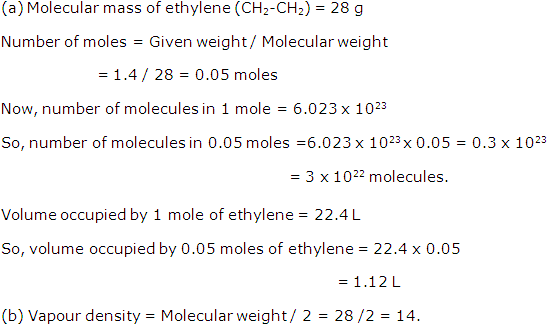
Question 4
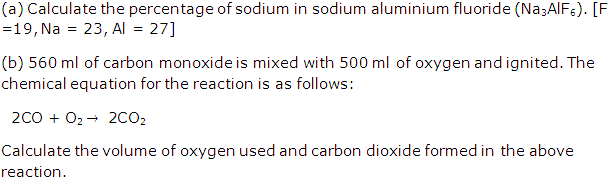
Solution 4

Question 5

Solution 5

Chapter 5 - Mole Concept And Stoichiometry Exercise 122
Question 1
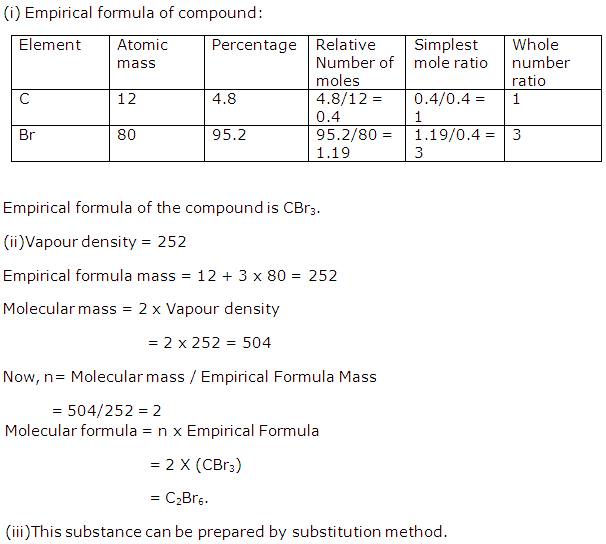
A compound 'X' consists of 4.8% of C and 95.2% of Br by mass.
(i) Determine the empirical formula of this compound. [C = 12, Br = 80]
(ii) If the vapour density of the compound is 252, what is the molecular formula of the compound?
(iii) Name the type of chemical reaction by which X can be prepared from ethane.
(i) Determine the empirical formula of this compound. [C = 12, Br = 80]
(ii) If the vapour density of the compound is 252, what is the molecular formula of the compound?
(iii) Name the type of chemical reaction by which X can be prepared from ethane.
Solution 1
Question 2
Choose the correct alternative:
The gas laws which relates the volume of a gas to the number of molecules of the gas is:
(a) Avogadro's law
(b) Gay-Lussac's law
(c) Boyle's law
(d) Charle's law
The gas laws which relates the volume of a gas to the number of molecules of the gas is:
(a) Avogadro's law
(b) Gay-Lussac's law
(c) Boyle's law
(d) Charle's law
Solution 2
The gas laws which relates the volume of a gas to the number of molecules of the gas is avogadro's law
Question 3
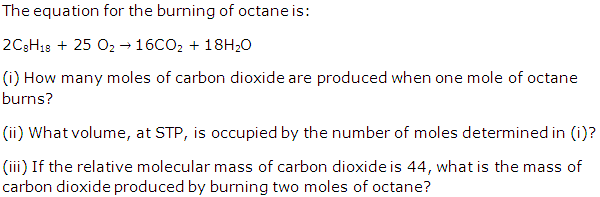
Solution 3
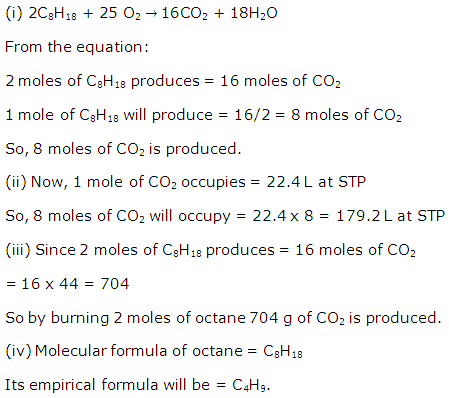
Question 4
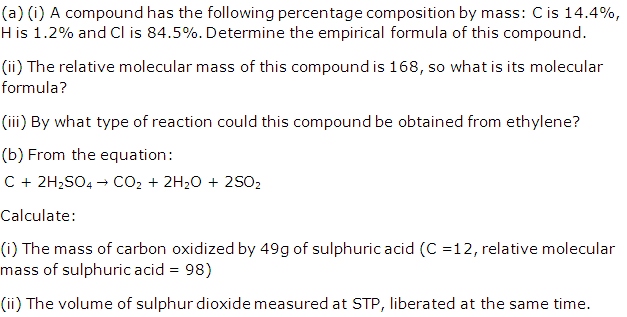
Solution 4
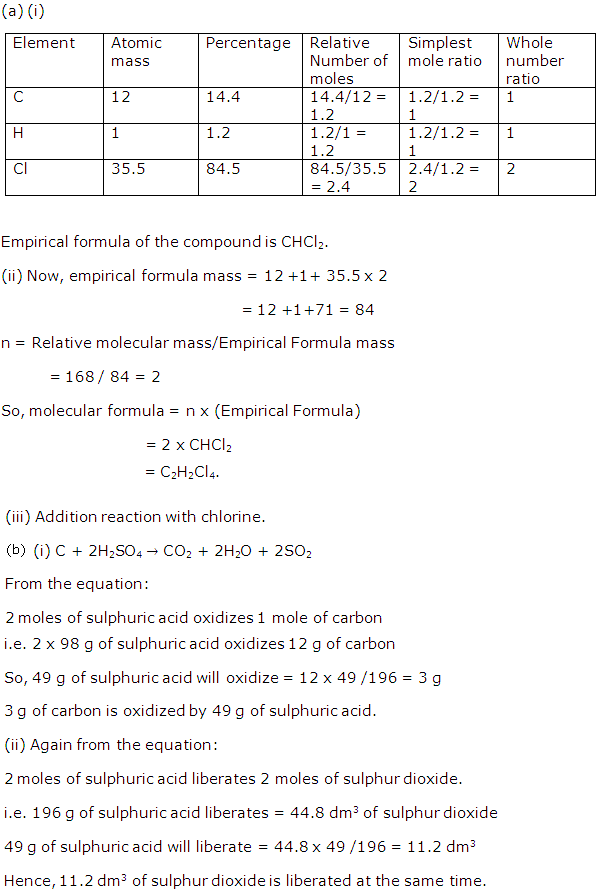
Chapter 5 - Mole Concept And Stoichiometry Exercise 123
Question 1
Choose the correct answer:
A gas cylinder of capacity of 20 dm3 is filled with gas X the mass of which is 10 g. When the same cylinder is filled with hydrogen gas at the same temperature and pressure the mass of the hydrogen is 2 g, hence the relative molecular mass of the gas is:
(a) 5
(b) 10
(c) 15
(d) 20
A gas cylinder of capacity of 20 dm3 is filled with gas X the mass of which is 10 g. When the same cylinder is filled with hydrogen gas at the same temperature and pressure the mass of the hydrogen is 2 g, hence the relative molecular mass of the gas is:
(a) 5
(b) 10
(c) 15
(d) 20
Solution 1
The relative molecular mass of the gas is 10.
Question 2
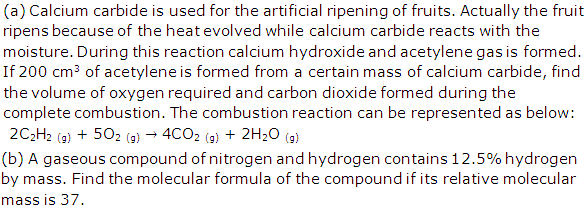
Solution 2
Question 3
Correct the following statement:
Equal masses of all gases under identical conditions contain the same number of molecules.
Equal masses of all gases under identical conditions contain the same number of molecules.
Solution 3
The correct statement is that equal volumes of all gases under identical conditions contain the same number of molecules.
Question 4

Solution 4
Question 5
(i) LPG stands for liquefied petroleum gas. Varieties of
LPG are marketed including a mixture of propane (60%) and butane (40%). If 10
litre of this mixture is burnt, find the total
volume of carbon dioxide gas added to the atmosphere. Combustion reaction can
be presented as :
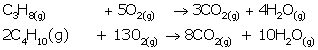
(ii) Calculate the percentage of nitrogen and
oxygen in ammonium nitrate [Relative molecular mass of ammonium nitrate is
80, H=1, N=14, O = 16].
Solution 5

(ii) Molecular mass of NH4(NO3) = 80
H = 1, N = 14, O = 16
% of nitrogen
As 80 g of NH4 (NO3)
contains 28 g of nitrogen,
% of nitrogen
As80 g of NH4(NO3)
contains48 g of oxygen
Question 6
4.5
moles of calcium carbonate are reacted with dilute hydrochloric acid.
(i) Write the equation for the reaction.
(ii) What is the mass of 4.5 moles of calcium carbonate?
Relative molecular mass of calcium carbonate is 100)
(iii) What is the volume of carbon dioxide liberated at STP?
(iv) What mass of calcium chloride is formed? (Relative
molecular mass of calcium chloride is 111)
(v) How many moles of HCl are
used in this reaction
Solution 6
(i) Equation for the reaction of calcium carbonate with
dilute hydrochloric acid:
(ii) Relative molecular mass of calcium carbonate=100
Mass of
4.5 moles of calcium carbonate
= No. of
moles× Relative molecular mass
= 4.5×100
= 450g
(iii) 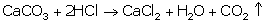
As100g of
calcium carbonate gives 22.4dm3 of CO2,
(iv) Molecular mass of calcium carbonate =100
Relative
molecular mass of calcium chloride =111
As 100 g
of calcium carbonate gives 111g of calcium chloride,
(v) Molecular mass of HCl=36.5
Molecular
mass of calcium carbonate =100
As 100 g
of calcium carbonate gives (2×36.5)= 73g of HCl,
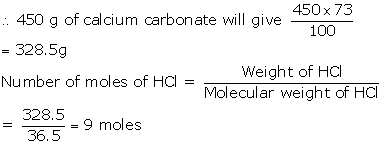
Chapter 5 - Mole Concept And Stoichiometry Exercise 124
Question 1
(i) Calculate the volume of 320 g of SO2 at STP
(Atomic mass : S = 32 and O = 16)
(ii) State Gay-Lussac's Law of combining volumes.
(iii) Calculate the volume of oxygen required for the
complete combustion of 8.8 of propane (C3H5). (Atomic
mass : C = 14, O = 16, H = 1, Molar Volume = 22.4 dm3 at STP)
Solution 1
(i) Atomic mass: S = 32 and O = 16
Molecular
mass of SO2=32+(2×16)=64g
As 64 g of
SO2 = 22.4dm3,
(ii) Gay-Lussac's law: When gases react, they do so in
volumes which bear a simple ratio to one another and to the volume of the
gaseous product, if all the volumes are measured at the same temperature and
pressure.
(iii) C3H8 + 5O2 → 3CO2 + 4H2O
Molar mass
of propane = 44
44 g of
propane requires 5 × 22.4 litres of oxygen at STP.
Question 2
An
organic compound with vapour density = 94 contains
C = 12.67%, H = 2.13% and Br = 85.11%. Find the molecule formula.
[Atomic
mass C = 12, H = 1, Br = 80]
Solution 2
Element
|
Relative atomic mass
|
% Compound
|
Atomic ratio
|
Simplest ratio
|
H
|
1
|
2.13
|
2.13/1 = 2.13
|
2
|
C
|
12
|
12.67
|
12.67/12 = 1.055
|
2
|
Br
|
80
|
85.11
|
85.11/80 = 1
|
1
|
Empirical
formula = CH2Br
n(Empirical
formula mass of CH2Br) = Molecular mass (2 × VD)
n(12
+ 2 + 80) = 94 × 2
n
= 2
Molecular
formula = Empirical formula × 2
=
(CH2Br) × 2
=
C2H4Br2
Question 3
Calculate
the mass of
(i) 1022 atoms of sulphur
(ii) 0.1 mole of carbon dioxide
[Atomic
mass : S = 32,C=12, and O = 16 and Avogadro's number = 6 × 1023]
Solution 3
(i) 1022 atoms of sulphur
6.022 × 1023
atoms of sulphur will have mass = 32 g
(ii) 0.1 mole of carbon dioxide
1 mole of
carbon dioxide will have mass = 44 g
0.1 mole of
carbon dioxide will have mass = 4.4 g
Question 4
O2is
evolved by heating KCIO3 using MnO2 as a catalyst.
(i) Calculate the mass of KClO3
required to produce 6.72 litre of O2at
STP
[Atomic
masses : K = 39, Cl = 35.5, O = 16]
(ii) Calculate the number of moles of oxygen present in the
above volume and also the number of molecules
(iii) Calculate the volume occupied by 0.01 mole of CO2at
STP
Solution 4

Question 5
Solve
the following :
(i) What volume of oxygen is required to burn completely
90dm3 of butane under similar conditions of temperature and
pressure?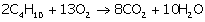
(ii) The vapour density of a gas
is 8. What would be the volume occupied by 24.0 of the gas at STP?
(iii) A vessel contains X number of molecules of hydrogen gas
at a certain temperature and pressure. How many molecules of nitrogen gas
would be present in the same vessel under the same conditions of temperature
and pressure?
Solution 5

Question 6
(i) Oxygen oxidase ethyne to carbon dioxide and water as shown by the
equation :
2C2H2
+ 5O2 → 4CO2 + 2H2O
What
volume of ethylene gas at STP is required to produce 8.4 dm3of
carbon dioxide at STP?
(ii) A compound made up of two elements X and Y
has an empirical formula X2Y. if the
atomic weight of X is 10 and that Y is 5 and the compound has a vapour density 25, find its molecular formula.
Solution 6
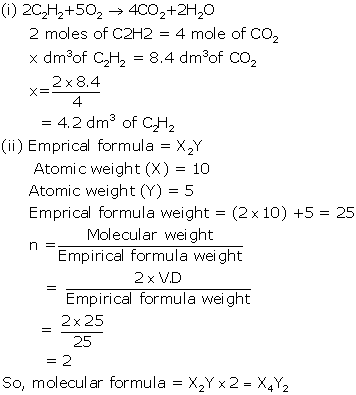
Chapter 5 - Mole Concept And Stoichiometry Exercise 125
Question 1
Give
one word or phrase for the following:
(i) The ratio of the mass of a certain volume of gas to the
mass of an equal volume of hydrogen under the same conditions of temperature
and pressure.
(ii) Formation of ions from molecules
Solution 1
(i) Vapour density
(ii) Ionisation
Question 2
(i) State Avogadro's Law
(ii) A cylinder contains 68g of ammonia gas at STP
(1) What is the volume occupied by this gas?
(2) How many moles of ammonia are present in the cylinder?
(3) How many molecules of ammonia are present in the
cylinder? [N-14,H-1]
Solution 2
(i) Avogadro's law:Equal
volumes of all gases under similar conditions of temperature and pressure
contain the same number of molecules.
(ii)

Question 3
Which
of the following would weigh the least?
(A) 2 gram atoms of Nitrogen
(B) 1 mole of Silver
(C) 22.4 litres of
oxygen gas at 1 atmospheric pressure and 273K
(D) 6.02 × 1023 atoms of carbon
[Atomic
masses : Ag = 108, N - 14, O = 16,C = 12]
Solution 3
6.02 × 1023 atoms of carbon
Question 4
Complete
the following calculations. Show working for complete credit:
(i) Calculate the mass of calcium that will
contain the same number of atoms as are present in 3.2 gm of Sulphur. [Atomic masses : S = 32, Ca = 40]
(ii) If 6 litres of
hydrogen and 4 litres of chlorine are mixed and
exploded and if water is added to the gases formed, find the volume of the
residual gas.
(iii) If the empirical formula of a compound is CH and it has
a vapour density of 13, find the molecular formula
of the compound.
Solution 4
(i) 3.2 g of S has number of atoms = 6.023 × 1023 x 3.2 /32
=
0.6023 × 1023
So, 0.6023 × 1023 atoms of Ca has mass=40 × 0.6023 ×1023/6.023 × 1023 = 4g
(ii) 6 litres of hydrogen and 4 litres of chlorine when mixed result in the formation of
8 litres of HCl gas.
When
water is added to it, it results in the formation of hydrochloric acid.
Chlorine acts as a limiting agent leaving behind only 2 litres
of hydrogen gas.
Therefore,
the volume of the residual gas will be 2 litres.
(iii)
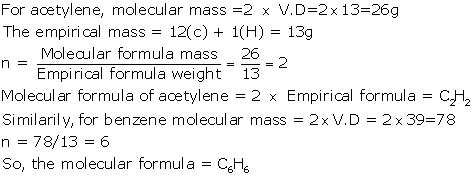
Question 5
Consider
the following reaction and based on the reaction answer the questions that
follow :
Calculate
:
(i) The quantity in moles of (NH4)2Cr2O7
if 63 gm of (NH4)2Cr2O7 is heated.
(ii) The quantity in moles of nitrogen formed.
(iii) The volume in litres or dm3
of N2evolved at STP
(iv) The mass in grams of Cr2O3 formed
at the same time
[Atomic
masses : H = 1, Cr=52, N=14]
Solution 5
(i)

(ii) 252 g of ammonium dichromate gives 22.4 dm3of
N2
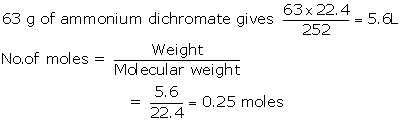
(iii)

(iv) 252 g of ammonium dichromate gives 152 g of CrO3
Question 6
A
gas cylinder contain 12 × 1024 molecules of oxygen gas
If
Avogadro's number is 6 × 1023, calculate :
(i) The mass of oxygen present in the cylinder
(ii) The volume of oxygen at STP present in the
Cylinder [O = 16]
Solution 6
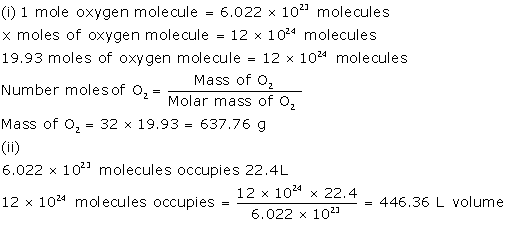
Question 7
A
gaseous hydrocarbon contains 82.76% of carbon. Given that its vapour density is 29, find its molecular formula.
[C
= 12, H = 1]
Solution 7
% of
carbon = 82.76%
% of
hydrogen = 100 - 82.76 = 17.24%
Element
|
% Weight
|
Atomic
Weight
|
Relative
No. of
moles
|
Simplest
Ratio
|
C
|
82.76
|
12
|
82.76/12 =
6.89
|
6.89/6.8
= 1 × 2 = 2
|
H
|
17.24
|
1
|
17.24/1 =
17.24
|
17.24/6.89
= 2.5 × 2 = 5
|
Empirical formula = C2H5
Empirical
formula weight = 2 × 12 + 1 × 5 = 24 +
5 = 29
Vapour density =
29
Relative
molecular mass = 29 × 2 = 58
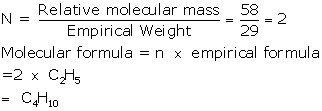
Chapter 5 - Mole Concept And Stoichiometry Exercise 126
Question 1
The
equation 4NH35O2 → 4NO + 6H2O represents the
catalytic oxidation of ammonia. If 100 cm3of
ammonia is used, calculate the volume of oxygen required to oxidise the ammonia completely.
Solution 1
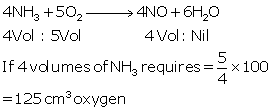
Question 2
A
gas of 32 g mass has a volume of 20 litres at STP.
Calculate the gram molecular weight of the gas.
Solution 2
Mass of
gas = 32 g
Volume
occupied by 32g of gas = 20litres
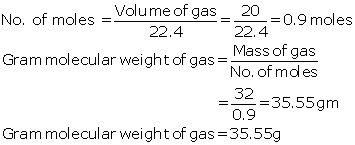
Question 3
How
much calcium oxide is formed when 82 g of calcium nitrate is heated? Also
find the volume of nitrogen dioxide evolved:
2Ca(NO3)2→
2CaO + 4NO2 + O2 (Ca = 40,N = 14, O = 16)
Solution 3
2Ca(NO3)2→ 2CaO + 4NO2 + O2
Molecular
weight of 2Ca(NO3)2 = 2[40+2(14+48) = 32g
Molecular
weight of CaO = 2(40 + 16) = 112g
328 g of
Ca(No3]2 liberates 4 moles of NO2
328 g of
Ca (NO3)2 liberates 4 × 22.4 L of
NO2
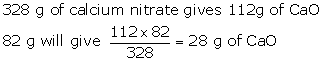
Question 4
The
ratio between the number of molecules in 2g of hydrogen and 32 g of oxygen is
(A) 1:2
(B) 1:0:01
(C) 1:1
(D) 0.01:1
[Given that H=1,O=16]
Solution 4
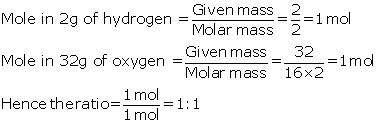
Question 5
(a) Calculate the number of gram atoms in 4.6
grams of sodium (Na = 23)
(b) Calculate the percentage of water of
crystallization in CuSO4. 5H2O
(H
= 1, O = 16, S = 32, Cu = 64)
(c) A compound of X and Y has the empirical formula XY2.
Its vapour density is equal to its empirical
formula weight. Determine its molecular formula.
Solution 5
(a) 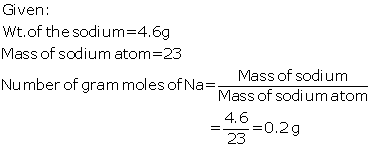
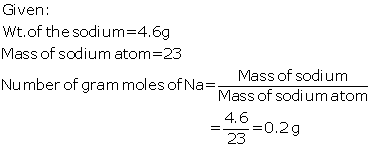
(b) 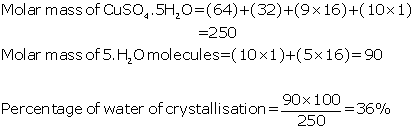
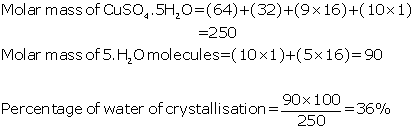
(c) 


Question 6
(a) Propane burns in air according to the
following equation :
C3H8
+ 5O2 → 3CO2 + 4H2O
What
volume of propane is consumed on using 1000 cm3of air, considering
only 20% of air contains oxygen?
(b) The mass of 11.2 litres of a certain gas at s.t.p is 24 g. Find gram molecular mass of the gas
Solution 6
(a)
Given:
C3H8
+ 5O2 → 3CO2 + 4H2O
Volume
of air = 1000 cm3
Percentage
of oxygen in air = 20%
From
the given information,
According to Gay-Lussac's law,
1 vol. of propane consumes 5 vol. of oxygen.
Volume of oxygen = 1000 cm3 × 20% = 200
cm3
Therefore,
Volume of propane burnt for every 200 cm3
of oxygen,
40 cm3of propane is burnt.
(b)
(i) Given:
Volume
of gas at STP = 11.2 litres
Mass of gas at STP = 24 g
Gram molecular mass = ?
Mass of 22.4 L of a gas at STP is equal to its gram
molecular mass.
11.2 L of the gas at STP weighs 24 g
So,
22.4 L of the gas will weigh
Gram molecular mass = 48 g
Question 7
A
gas cylinder can hold 1 kg of hydrogen at room temperature and pressure.
(a) Find the number of moles of hydrogen
present
(b) What weight of CO2can the
cylinder hold under similar conditions of temperature and pressure? (H = 1 C
= 12, O = 16)
(c) If the number of molecules of hydrogen in the cylinder
is X, calculate the number of CO2 molecules in the cylinder with
the same conditions of temperature and pressure.
(d) State the law that helped you to arrive at
the above result
Solution 7
(a)
Given:
Mass of hydrogen = 1 kg at 298 K and 1 atm pressure
Moles of hydrogen =?
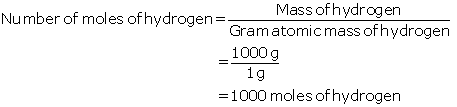
(b) Molecular mass of CO2 = 12 + 2 × 16 = 44g
So, vapour
density (VD) = mol. Mass/2 = 44/2 = 22
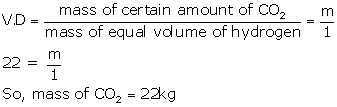
(c) According to Avogadro's law, equal volumes of all gases
under similar conditions of temperature and pressure contain equal number of
molecules.
(d) So, number of molecules of carbon dioxide
in the cylinder = number of molecules of hydrogen in the cylinder = X

0 comments:
Post a Comment