Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 16
Question 1
What is modern periodic law?
Solution 1
Modern periodic law states that the physical and chemical properties of elements are a periodic function of their atomic numbers i.e., if the elements are arranged in the order of their atomic numbers, the elements with similar properties are repeated after definite regular intervals.
Concept Insight: The elements are characterized by their atomic number as well as atomic weight. Modern periodic law uses atomic number which is number of protons or number of electrons present in an atom of an element.
Concept Insight: The elements are characterized by their atomic number as well as atomic weight. Modern periodic law uses atomic number which is number of protons or number of electrons present in an atom of an element.
Question 2
How many groups and periods are there in the modern periodic table?
Solution 2
Modern periodic table consists of eighteen groups and seven periods.
Concept Insight: Classification of elements on the basis of increasing atomic number is known as Modern Periodic Table. The vertical columns are called groups and the horizontal rows are called periods.
Concept Insight: Classification of elements on the basis of increasing atomic number is known as Modern Periodic Table. The vertical columns are called groups and the horizontal rows are called periods.
Question 3
What is meant by periodicity of elements?
Solution 3
The recurrence of similar properties of elements after certain regular intervals when they are arranged in the order of increasing atomic numbers is called periodicity.
Concept Insight: Periodicity in properties is due to the repetition of similar outer electronic configuration of elements at certain regular intervals.
Concept Insight: Periodicity in properties is due to the repetition of similar outer electronic configuration of elements at certain regular intervals.
Question 4
In terms of electronic configuration, what the elements of a given period and groups have in common?
Solution 4
In general, the elements belonging to a group have the same number of valence electrons .For example, all the group 1 elements have valency one since they have only one electron in their outermost shell.
In general, the elements belonging to a period do not have same valency but their valence shell remains the same. For example, second period has 8 elements with atomic number 3 to 10 but in all of them the valence electrons are present in shell number two.
Concept Insight: For elements in a group the number of electrons present in the outermost shell is the same and therefore the elements have same valency and or elements in a period number of electrons present in the outermost shell of elements in a period increase from left to right but the shell does remains the same.
In general, the elements belonging to a period do not have same valency but their valence shell remains the same. For example, second period has 8 elements with atomic number 3 to 10 but in all of them the valence electrons are present in shell number two.
Concept Insight: For elements in a group the number of electrons present in the outermost shell is the same and therefore the elements have same valency and or elements in a period number of electrons present in the outermost shell of elements in a period increase from left to right but the shell does remains the same.
Question 5
Why is the electron affinity of fluorine less than chlorine?
Solution 5
Fluorine has lower electron affinity than chlorine because of the small size of fluorine which results in stronger repulsion between the electron and the electrons already present in the atom of fluorine. Hence the energy released in accepting an electron is lesser in fluorine than that of chlorine.
Concept Insight: For answering this question you should recall that electron affinity is the energy released when an electron is added to an isolated gaseous atom to form an anion. Due to small size of fluorine atom, the valence shell is already crowded, hence when an electron is added to a fluorine atom in gaseous state there occurs strong repulsion between the added electron and those already present in the atom hence less amount of energy is released.
Concept Insight: For answering this question you should recall that electron affinity is the energy released when an electron is added to an isolated gaseous atom to form an anion. Due to small size of fluorine atom, the valence shell is already crowded, hence when an electron is added to a fluorine atom in gaseous state there occurs strong repulsion between the added electron and those already present in the atom hence less amount of energy is released.
Question 6
Among the elements of the second period, Li to Ne, pick out the element:
(i) With highest first ionization energy
(ii) With the highest electro negativity
(iii) With the largest atomic size
(iv) That is the most reactive non-metal
(v) That is the most reactive metal
(i) With highest first ionization energy
(ii) With the highest electro negativity
(iii) With the largest atomic size
(iv) That is the most reactive non-metal
(v) That is the most reactive metal
Solution 6
(i) The element with highest first ionization energy: Neon (Ne)
(ii) The element with highest electro negativity: Fluorine (F)
(iii) The element with largest atomic size: Lithium (Li)
(iv) The most reactive non-metal: Fluorine
(v) The most reactive metal: Lithium
Concept Insight: (i) Neon has highest ionization energy since it is a noble gas and has its octet complete which makes it very stable.
(ii) Fluorine has highest electronegativity as we know that electro negativity increases along a period due to decreasing atomic size and increasing nuclear charge.
(iii) Lithium has the largest atomic size since it is an alkali metal i.e., belongs to group 1 and we know that as we move from left to right in a period atomic size decreases. So lithium has largest size while fluorine has smallest size in second period.
(iv) Fluorine is most reactive non metal as it requires only one electron to complete its octet and become stable.
(vi) Lithium is the most reactive metal as it can complete its octet by losing its single electron present in its outermost shell.
(ii) The element with highest electro negativity: Fluorine (F)
(iii) The element with largest atomic size: Lithium (Li)
(iv) The most reactive non-metal: Fluorine
(v) The most reactive metal: Lithium
Concept Insight: (i) Neon has highest ionization energy since it is a noble gas and has its octet complete which makes it very stable.
(ii) Fluorine has highest electronegativity as we know that electro negativity increases along a period due to decreasing atomic size and increasing nuclear charge.
(iii) Lithium has the largest atomic size since it is an alkali metal i.e., belongs to group 1 and we know that as we move from left to right in a period atomic size decreases. So lithium has largest size while fluorine has smallest size in second period.
(iv) Fluorine is most reactive non metal as it requires only one electron to complete its octet and become stable.
(vi) Lithium is the most reactive metal as it can complete its octet by losing its single electron present in its outermost shell.
Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 17
Question 1
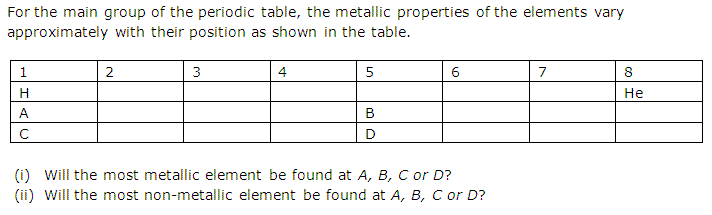
Solution 1
(i) The most metallic element will be found at C.
(ii) The most non-metallic element will be found at D.
Concept Insight: For answering this question you should recall metallic character increases down the group and also increases with the increasing size of the atom. Since elements of group 1 has largest atomic sizes among all the elements of periodic table so the most metallic element belongs to group 1.
Similarly, non-metallic character decreases down the group and increases with the decreasing size of atom.
(ii) The most non-metallic element will be found at D.
Concept Insight: For answering this question you should recall metallic character increases down the group and also increases with the increasing size of the atom. Since elements of group 1 has largest atomic sizes among all the elements of periodic table so the most metallic element belongs to group 1.
Similarly, non-metallic character decreases down the group and increases with the decreasing size of atom.
Question 2
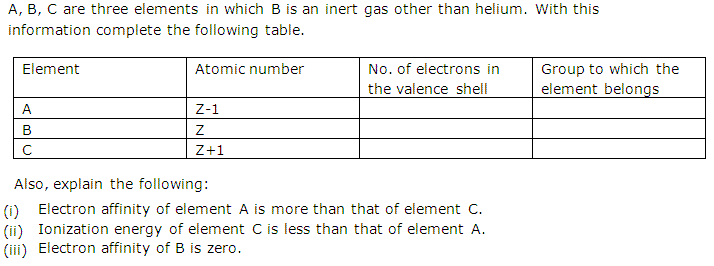
Solution 2
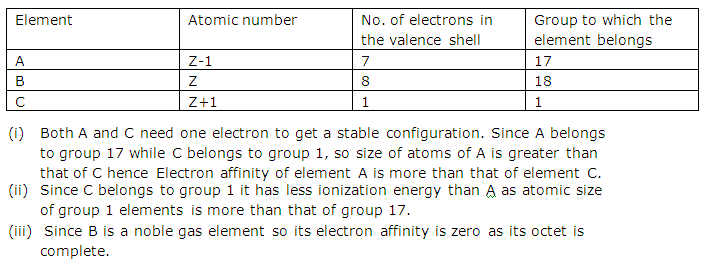
Question 3
Fill in the blanks:
(i) There are ______ groups and ______ periods in the modern form of periodic table.
(ii) Most electropositive elements belong to ______ group.
(iii) Most electronegative elements belong to ______ group.
(iv) Energy released when electron is added to a neutral gaseous atom is called ______.
(v) Size of the atoms ______ from left to right across a period and ______ on descending in a group of normal elements.
(vi) The maximum electro negativity is shown by ______.
(vii) Noble gases have ______ electron affinity.
(viii) Oxidising property ________from fluroine to iodine because the power to accpet electron decreases.
(ix) Reactivity ______ down the gruop for alkali metals with increase in electropositive character
(x) The nature of oxide Al2O3 is ____.
(i) There are ______ groups and ______ periods in the modern form of periodic table.
(ii) Most electropositive elements belong to ______ group.
(iii) Most electronegative elements belong to ______ group.
(iv) Energy released when electron is added to a neutral gaseous atom is called ______.
(v) Size of the atoms ______ from left to right across a period and ______ on descending in a group of normal elements.
(vi) The maximum electro negativity is shown by ______.
(vii) Noble gases have ______ electron affinity.
(viii) Oxidising property ________from fluroine to iodine because the power to accpet electron decreases.
(ix) Reactivity ______ down the gruop for alkali metals with increase in electropositive character
(x) The nature of oxide Al2O3 is ____.
Solution 3
(i) 18, 7.
(ii) First.
(iii) Seventeen.
(iv) Electron affinity.
(v) Decrease, increase
(vi) Fluorine
(vii) Zero.
(ii) First.
(iii) Seventeen.
(iv) Electron affinity.
(v) Decrease, increase
(vi) Fluorine
(vii) Zero.
Question 4
State whether the following statements are true or false:
(i) Fluorine has higher electron affinity than chlorine.
(ii) Similar electronic configuration is repeated after intervals of 2, 8, 8, 18 and 32.
(iii) Al2O3 is an amphoteric oxide.
(iv) On moving horizontally across a period, number of valence electrons increases from one to eight.
(v) All members of zero group are non metals.
(vi) The elements with higher electron affinity have higher ionization potential.
(i) Fluorine has higher electron affinity than chlorine.
(ii) Similar electronic configuration is repeated after intervals of 2, 8, 8, 18 and 32.
(iii) Al2O3 is an amphoteric oxide.
(iv) On moving horizontally across a period, number of valence electrons increases from one to eight.
(v) All members of zero group are non metals.
(vi) The elements with higher electron affinity have higher ionization potential.
Solution 4
(i) False.
(ii) True.
(iii) True.
(iv) True.
(v) True.
(vi) True.
Concept Insight: (i) There is inverse relation between atomic size and electron affinity. More is the size, less is the electron affinity and vice versa because more is the size of atom, more is the distance between the nucleus and last shell to which electron enters. This results in decrease in force of attraction between the nucleus and incoming electron and hence the electron affinity decreases. Fluorine has smaller size than chlorine so it must have less electron affinity than chlorine.
(ii) True.
(iii) True.
(iv) True.
(v) True.
(vi) True.
Concept Insight: (i) There is inverse relation between atomic size and electron affinity. More is the size, less is the electron affinity and vice versa because more is the size of atom, more is the distance between the nucleus and last shell to which electron enters. This results in decrease in force of attraction between the nucleus and incoming electron and hence the electron affinity decreases. Fluorine has smaller size than chlorine so it must have less electron affinity than chlorine.
Question 5
Arrange the following in increasing order of property indicated:
(i) Li, Be, B (ionization energy)
(ii) F, Cl, Br, I (electron affinity)
(iii) SiO2, P2O5, SO3, Cl2O7 (acidic nature)
(iv) I, I+, I- (atomic size)
(i) Li, Be, B (ionization energy)
(ii) F, Cl, Br, I (electron affinity)
(iii) SiO2, P2O5, SO3, Cl2O7 (acidic nature)
(iv) I, I+, I- (atomic size)
Solution 5
(i) Li < Be < B
(ii) I < Br < F < Cl
(iii) SiO2 < P2O5 < SO3 < Cl2O7
(iv) I+ < I < I-
Concept Insight:
(i) Li, Be, B belongs to second period and ionization energy increase as we move left to right in a period due to increased nuclear charge and decrease of atomic size.
(ii) Electron affinity decreases down the group due to increase in atomic size as it results in more distance between nucleus and last shell to which incoming electron enters. Hence, incoming electron feels less attraction from the nucleus.
(iii) In a period, acidic nature of oxide increases.
(iv) Size of a cation is always smaller than the corresponding atom due to decrease in number of electrons and increase in effective nuclear charge i.e., greater force of attraction by the nucleus on the electrons.
Size of an anion is always more than the corresponding atom due to decrease in effective nuclear charge i.e., lesser force of attraction by the nucleus o the electrons.
(ii) I < Br < F < Cl
(iii) SiO2 < P2O5 < SO3 < Cl2O7
(iv) I+ < I < I-
Concept Insight:
(i) Li, Be, B belongs to second period and ionization energy increase as we move left to right in a period due to increased nuclear charge and decrease of atomic size.
(ii) Electron affinity decreases down the group due to increase in atomic size as it results in more distance between nucleus and last shell to which incoming electron enters. Hence, incoming electron feels less attraction from the nucleus.
(iii) In a period, acidic nature of oxide increases.
(iv) Size of a cation is always smaller than the corresponding atom due to decrease in number of electrons and increase in effective nuclear charge i.e., greater force of attraction by the nucleus on the electrons.
Size of an anion is always more than the corresponding atom due to decrease in effective nuclear charge i.e., lesser force of attraction by the nucleus o the electrons.
Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 18
Question 1
Explain, the statement 'In each period, the atomic radii gradually decrease with increase in atomic number'. Give one example to justify your answer.
Solution 1
The statement that in each period, the atomic size gradually decreases with increase in atomic number means that as move from left to right in a period, nuclear charge increases by one unit in each succeeding element while the number of shells remains the same. Due to this increased nuclear charge, the electrons of all the shells are pulled closer to the nucleus thereby bringing the outer most shell closer to the nucleus. With the result, the atomic size decreases across a period.
For example, in the second period from lithium to fluorine, lithium has the largest size while fluorine has the smallest size.
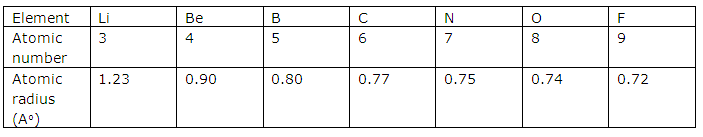
For example, in the second period from lithium to fluorine, lithium has the largest size while fluorine has the smallest size.

Question 2
How could the atomic radius of a noble gas be compared with the other elements in a period?
Solution 2
In the case of noble gases or inert gases there are exceptions and the atomic radius or size of the elements are greater than the other elements of the period to which these elements belong.
Question 3
'In a group, the atomic radii increase with increasing period number', explain this statement and justify it with reference to group 17.
Solution 3
As we move down a group, the atomic radii increase because a new shell is added at each succeeding element though the number of electrons in the outer most shell remains the same. Thus, the atomic size of elements increases in size downward.
Although nuclear charge also increases in going down the group but the effect of nuclear charge on atomic size is much less than the increase due to addition of a new shell.
In group 17, the atomic size follows the trend:
F < Cl < Br < I
Although nuclear charge also increases in going down the group but the effect of nuclear charge on atomic size is much less than the increase due to addition of a new shell.
In group 17, the atomic size follows the trend:
F < Cl < Br < I
Question 4
In the third period, which is the most metallic and most non-metallic element?
Solution 4
The elements of third period are:
Na, Mg, Al, Si, P, S, Cl, Ar
The most metallic element is sodium i.e., Na and the most non-metallic element is chlorine i.e., Cl.
Concept Insight:
In a period, metallic character decreases on moving from left to right because of decrease in size of atom due to which elements cannot lose electron easily.
Na, Mg, Al, Si, P, S, Cl, Ar
The most metallic element is sodium i.e., Na and the most non-metallic element is chlorine i.e., Cl.
Concept Insight:
In a period, metallic character decreases on moving from left to right because of decrease in size of atom due to which elements cannot lose electron easily.
Question 5
What is meant by ionization potential?
Solution 5

Question 6
In a group, a particular element has the lowest ionization potential; does it ionize most easily or least easily? Explain with example.
Solution 6
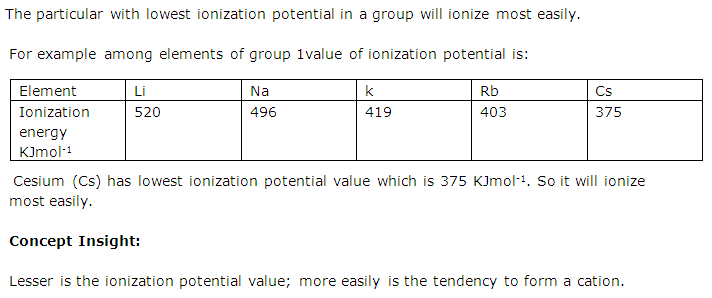
Question 7
What is electron affinity?
Solution 7
Electron affinity is the energy released when an electron is added to an isolated gaseous atom to form the negative ion (anion).
Unit: Its units are electron volt (eV).
Its SI units are Kilojoules per mole (KJmol-1).
Unit: Its units are electron volt (eV).
Its SI units are Kilojoules per mole (KJmol-1).
Question 8
Electron affinities of two elements A and B are given below:
A = 3.79 electron volts
B = 3.56 electron volts
Which of them will ionize more easily and why?
A = 3.79 electron volts
B = 3.56 electron volts
Which of them will ionize more easily and why?
Solution 8
Out of A and B, A will ionize more easily to form a negative anion because of the high value of electron affinity, energy released during addition of electron will be high hence the resulting anion formed will be more stable than the corresponding atom.
Question 9
Explain:
(i) Larger the atomic size, more metallic is the element.
(ii) Halogens have high electron affinity.
(iii) Size of atom changes when it loses or gain electron
(iv) K is more reactive than Li.
(v) Electronegativity of Cl is higher than S.
(vi) Group 17 elements are non-metals, while group 1 elements are metals.
(i) Larger the atomic size, more metallic is the element.
(ii) Halogens have high electron affinity.
(iii) Size of atom changes when it loses or gain electron
(iv) K is more reactive than Li.
(v) Electronegativity of Cl is higher than S.
(vi) Group 17 elements are non-metals, while group 1 elements are metals.
Solution 9
(i) Larger the atomic size, farther is the valence electron from the nucleus and lesser is the pull exerted on it. As a result, electron can be easily removed from the valence shell and hence more metallic is the element.
(ii) Halogens need only one electron to complete their octet and become stable their atomic size is very less hence the distance between their last shell and nucleus is very less, as a result the force of attraction between the nucleus and the incoming electron is less and hence the electron affinity is high for halogens.
(iii) When an atom loses or gain electron to form ion, the number of electrons present in the outermost shell also changes. Corresponding to that effective nuclear charge on the changed number of electrons also change which further changes the size of an atom as there is inverse relation between effective nuclear charge and size of atom.
(iv) K and Li belongs to group 1 i.e., metals and we know that for metals chemical reactivity of elements increases down the group because chemical reactivity increases as electropositive or metallic character increases.
(v) The electronegativity of chlorine is higher than sulphur because both of them belong to third group and chlorine follows sulphur. We know that, within a period electronegativity increases as we move from left to right because of decrease in atomic size and increase in nuclear charge.
(vi) Group 17 elements are non metals because they have 7 electrons in their valence shell and ionize by accepting 1 electron to form an anion.
For example group 17 elements F, Cl, Br and I all have 7 electrons in their valence shell and ionize by accepting 1 electron to form F-, Cl-, Br- and I-.
Group 1 elements are metals because they have tendency to lose the one electron present in their valence shell and form positive ion.
For example, group 1 elements Li, Na, K, Rb, Cs have tendency to lose the one electron present in their valence shell and form positive ions Li+, Na+, K+, Rb+ and Cs+.
(ii) Halogens need only one electron to complete their octet and become stable their atomic size is very less hence the distance between their last shell and nucleus is very less, as a result the force of attraction between the nucleus and the incoming electron is less and hence the electron affinity is high for halogens.
(iii) When an atom loses or gain electron to form ion, the number of electrons present in the outermost shell also changes. Corresponding to that effective nuclear charge on the changed number of electrons also change which further changes the size of an atom as there is inverse relation between effective nuclear charge and size of atom.
(iv) K and Li belongs to group 1 i.e., metals and we know that for metals chemical reactivity of elements increases down the group because chemical reactivity increases as electropositive or metallic character increases.
(v) The electronegativity of chlorine is higher than sulphur because both of them belong to third group and chlorine follows sulphur. We know that, within a period electronegativity increases as we move from left to right because of decrease in atomic size and increase in nuclear charge.
(vi) Group 17 elements are non metals because they have 7 electrons in their valence shell and ionize by accepting 1 electron to form an anion.
For example group 17 elements F, Cl, Br and I all have 7 electrons in their valence shell and ionize by accepting 1 electron to form F-, Cl-, Br- and I-.
Group 1 elements are metals because they have tendency to lose the one electron present in their valence shell and form positive ion.
For example, group 1 elements Li, Na, K, Rb, Cs have tendency to lose the one electron present in their valence shell and form positive ions Li+, Na+, K+, Rb+ and Cs+.
Question 10
Select the correct answer:
(i) Which of the following electronic structure is of a metal?
(a) 2, 8, 8
(b) 2, 7
(c) 2, 8, 2
(d) 2, 8, 4
(ii) Atomic radii of fluorine and neon in angstrom unit are
(a) 0.72, 13.6
(b) 1.60, 1.60
(c) 0.72, 0.72
(d) none of these
(iii) Configuration of an element is 2, 8, 1. Which of the following statement is true?
(a) Valency of element is 7
(b) Element is diatomic
(c) Element is non metal
(d) Element forms basic oxide
(iv) Which of the following element has highest electronegativity?
(a) F
(b) H
(c) Ne
(d) Na
(v) Number of shells in potassium are
(a) 3
(b) 2
(c) 4
(d) 5
(vi) Most reactive character among the elements given below is found in
(i) I
(ii) Br
(iii) Cl
(iv) F
(vii) Identify the metalloid
(a) N
(b) P
(c) Bi
(d) Sb
(i) Which of the following electronic structure is of a metal?
(a) 2, 8, 8
(b) 2, 7
(c) 2, 8, 2
(d) 2, 8, 4
(ii) Atomic radii of fluorine and neon in angstrom unit are
(a) 0.72, 13.6
(b) 1.60, 1.60
(c) 0.72, 0.72
(d) none of these
(iii) Configuration of an element is 2, 8, 1. Which of the following statement is true?
(a) Valency of element is 7
(b) Element is diatomic
(c) Element is non metal
(d) Element forms basic oxide
(iv) Which of the following element has highest electronegativity?
(a) F
(b) H
(c) Ne
(d) Na
(v) Number of shells in potassium are
(a) 3
(b) 2
(c) 4
(d) 5
(vi) Most reactive character among the elements given below is found in
(i) I
(ii) Br
(iii) Cl
(iv) F
(vii) Identify the metalloid
(a) N
(b) P
(c) Bi
(d) Sb
Solution 10
(i) (C) i.e. 2, 8, 2 because it has only 2 electrons in its valence shell which can be lost to form a di positive cation.
(ii) (C) i.e. 0.72, 0.72
(iii) (d) i.e. element forms basic oxide because the element is a metal as it has valency 1.
(iv) (a) i.e. F because it belongs to group 17 whose elements have valency 7 and thus requires only 1 electron to complete their octet.
(ii) (C) i.e. 0.72, 0.72
(iii) (d) i.e. element forms basic oxide because the element is a metal as it has valency 1.
(iv) (a) i.e. F because it belongs to group 17 whose elements have valency 7 and thus requires only 1 electron to complete their octet.
Question 11
(i) State the number of elements in period 1, period 2 and period 3 of the periodic table.
(ii) Name the elements in period 1.
(iii) What happens to the atomic size of elements on moving from left to right in a period?
(ii) Name the elements in period 1.
(iii) What happens to the atomic size of elements on moving from left to right in a period?
Solution 11
(i) Number of elements in period 1 = 2
In period 2 = 8
In period 3 = 8.
(ii) Elements in period 1 are Hydrogen (H) and Helium (He).
(iii) Atomic size of elements decreases on moving from left to right in a period.
In period 2 = 8
In period 3 = 8.
(ii) Elements in period 1 are Hydrogen (H) and Helium (He).
(iii) Atomic size of elements decreases on moving from left to right in a period.
Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 19
Question 1
(i) What is the common feature of electronic configurations of the elements at the end of period 2 and period 3?
(ii) If an element is in group 7 is it likely to be metallic or non metallic in character?
(iii) Supply the missing word from the words given in brackets.
If an element has one electron in its outermost energy level, then it is likely to be _ (metallic, non metallic)
(ii) If an element is in group 7 is it likely to be metallic or non metallic in character?
(iii) Supply the missing word from the words given in brackets.
If an element has one electron in its outermost energy level, then it is likely to be _ (metallic, non metallic)
Solution 1
(i) The elements at the end of period 2 and period 3 both have their outermost shell complete and belong to noble gases.
(ii) An element in group 7 is likely to be non metallic in character since group 7 element will have 7 electrons in its valence shell.
(iii) Metallic.
(ii) An element in group 7 is likely to be non metallic in character since group 7 element will have 7 electrons in its valence shell.
(iii) Metallic.
Question 2
Copy and complete the following sentences choosing the correct word or words from those given in the brackets, at the end of each sentence:
1. The properties of the elements are a periodic function of their ______ (atomic number, mass number, relative atomic mass).
2. Moving across a ______ of the periodic table, the elements show increasing ______ character.(group, period, metallic, non metallic).
3. The element at the bottom of a group would be expected to show ______ metallic character than the element at the top (less, more).
4. The similarities in the properties of elements belonging to a group are because they have the same ______ (electronic configuration, number of outer electrons, atomic number).
1. The properties of the elements are a periodic function of their ______ (atomic number, mass number, relative atomic mass).
2. Moving across a ______ of the periodic table, the elements show increasing ______ character.(group, period, metallic, non metallic).
3. The element at the bottom of a group would be expected to show ______ metallic character than the element at the top (less, more).
4. The similarities in the properties of elements belonging to a group are because they have the same ______ (electronic configuration, number of outer electrons, atomic number).
Solution 2
1. Atomic number.
2. Period, non-metallic.
3. More.
4. Number of outer electrons.
2. Period, non-metallic.
3. More.
4. Number of outer electrons.
Question 3
What is meant by a group in the periodic table?
Solution 3
A group is a vertical column of elements having the same number of valence electrons and same valency in the periodic table. There are 18 groups in the periodic table.
Question 4
Within a group, where would you expect to find the element with:
(i) The greatest metallic character.
(ii) The largest atomic size.
(i) The greatest metallic character.
(ii) The largest atomic size.
Solution 4
Within a group the element with the greatest metallic character and largest size is expected to be present at the bottom of the group.
Question 5
State whether the ionization potential increases or decreases on going down a group.
Solution 5
Ionization potential decreases down the group because atomic size increases down the group which decreases the effective nuclear charge over the valence electron which further can now be removed easily.
Question 6
How many elements are there in period 2?
Solution 6
There are 8 elements in period 2.
Question 7
The following table represents the first period of the modern periodic table. Study the table and answer the questions that follow:
(i) Write the formula of the sulphate of the element with atomic number 13.
(ii) What type of bonding will be present in the oxide of the element with atomic number 1?
(iii) Which feature of the atomic structure accounts for the similarities in the chemical properties of the elements in group VIIA of the periodic table?
(iv) Name the element which has the highest ionization potential.
(v) How many electrons are present in the valence shell of the element with atomic number 18?
(vi) What is the name given to the energy released, when an atom in its isolated gaseous state accepts an electron to form an anion?
(vii) What is the electronic configuration of the element in the third period which gains one electron to become an anion?
(viii) Fill in the blanks:
The atomic size ______ as we move from left to right across the period, because the ______ increases, but the ______ remains the same.
(i) Write the formula of the sulphate of the element with atomic number 13.
(ii) What type of bonding will be present in the oxide of the element with atomic number 1?
(iii) Which feature of the atomic structure accounts for the similarities in the chemical properties of the elements in group VIIA of the periodic table?
(iv) Name the element which has the highest ionization potential.
(v) How many electrons are present in the valence shell of the element with atomic number 18?
(vi) What is the name given to the energy released, when an atom in its isolated gaseous state accepts an electron to form an anion?
(vii) What is the electronic configuration of the element in the third period which gains one electron to become an anion?
(viii) Fill in the blanks:
The atomic size ______ as we move from left to right across the period, because the ______ increases, but the ______ remains the same.

Solution 7
(i) Al2(SO4)3.
(ii) Covalent.
(iii) The elements of group VIIA all have same number of electrons in their valence shell and same valency.
(iv) Neon
(v) 8 electrons are present in the valence shell of the element with atomic number 18.
(vi) Electron affinity.
(vii) Electronic configuration of element in the third period which gains one electron to become an anion is 2, 8, 7.
(viii) Decreases, number of valence shell electrons/outermost shell electrons, valence shell/ outermost shell.
(ii) Covalent.
(iii) The elements of group VIIA all have same number of electrons in their valence shell and same valency.
(iv) Neon
(v) 8 electrons are present in the valence shell of the element with atomic number 18.
(vi) Electron affinity.
(vii) Electronic configuration of element in the third period which gains one electron to become an anion is 2, 8, 7.
(viii) Decreases, number of valence shell electrons/outermost shell electrons, valence shell/ outermost shell.
Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 20
Question 1
The electro negativities (according to Pauling) of the elements in period 3 of the periodic table are as follows.
The elements are arranged in alphabetical order:
Al Cl Mg Na P S Si
1.5 3.0 1.2 0.9 2.1 2.5 1.8
(i) Arrange the elements in the order in which they occur in the periodic table from left to right. (The group 1 elements first, followed by the group 2 element and so on, up to group 7.)
(ii) Choose the word or phrase from the brackets which correctly completes each of the following statements:
(a) The element below sodium in the same group would be expected to have a ______ (lower/higher) electro negativity than sodium and the element above chlorine which would be expected to have a ______ (lower / higher) ionization potential than chlorine.
(b) On moving from left to right in a given period, the number of shells ______ (remains the same / decreases/ increases).
(c) On moving down a group, the number of valence electrons ______ (remains the same / increases/ decreases).
The elements are arranged in alphabetical order:
Al Cl Mg Na P S Si
1.5 3.0 1.2 0.9 2.1 2.5 1.8
(i) Arrange the elements in the order in which they occur in the periodic table from left to right. (The group 1 elements first, followed by the group 2 element and so on, up to group 7.)
(ii) Choose the word or phrase from the brackets which correctly completes each of the following statements:
(a) The element below sodium in the same group would be expected to have a ______ (lower/higher) electro negativity than sodium and the element above chlorine which would be expected to have a ______ (lower / higher) ionization potential than chlorine.
(b) On moving from left to right in a given period, the number of shells ______ (remains the same / decreases/ increases).
(c) On moving down a group, the number of valence electrons ______ (remains the same / increases/ decreases).
Solution 1
(i) Na Mg Al Si P S Cl.
(ii) (a) lower, higher.
(b) remains the same.
(c) remains the same.
(ii) (a) lower, higher.
(b) remains the same.
(c) remains the same.
Question 2
Parts (i) to (v) refer to changes in the properties of elements on moving from left to right across a period of the periodic table. For each property, choose the letter corresponding to the correct answer from the choices (a), (b), (c) and (d).
(i) The non metallic character of the elements:
(a) Decreases
(b) Increases
(c) Remains the same
(d) Depends on the period
(ii) The electro negativity
(a) Depends on the number of valence electrons
(b) Remains the same
(c) Decreases
(d) Increases
(iii) The ionization potential
(a) Goes up and down
(b) Decreases
(c) Increases
(d) Remains the same
(iv) The atomic size
(a) Decreases
(b) Increases
(c) Remains the same
(d) Sometimes increases and sometimes decreases
(v) The electron affinity of the elements in groups 1 to 7:
(a) Goes up and down
(b) Decreases and then increases
(c) Increases
(d) decreases
(i) The non metallic character of the elements:
(a) Decreases
(b) Increases
(c) Remains the same
(d) Depends on the period
(ii) The electro negativity
(a) Depends on the number of valence electrons
(b) Remains the same
(c) Decreases
(d) Increases
(iii) The ionization potential
(a) Goes up and down
(b) Decreases
(c) Increases
(d) Remains the same
(iv) The atomic size
(a) Decreases
(b) Increases
(c) Remains the same
(d) Sometimes increases and sometimes decreases
(v) The electron affinity of the elements in groups 1 to 7:
(a) Goes up and down
(b) Decreases and then increases
(c) Increases
(d) decreases
Solution 2
(i) b
(ii) d
(iii) c
(iv) a
(v) c
(ii) d
(iii) c
(iv) a
(v) c
Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 21
Question 1
The elements of one short period of the periodic table are given below in order from left to right:
Li Be B C O F Ne
(a) To which period do these elements belong?
(b) One element of this period is missing. Which is the missing element and where should it be placed?
(c) Place the three elements, fluorine, beryllium and nitrogen, in the order of increasing electro negativity.
(d) Which one of the above elements belongs to the halogen series?
Li Be B C O F Ne
(a) To which period do these elements belong?
(b) One element of this period is missing. Which is the missing element and where should it be placed?
(c) Place the three elements, fluorine, beryllium and nitrogen, in the order of increasing electro negativity.
(d) Which one of the above elements belongs to the halogen series?
Solution 1
(a) Second period.
(b) Nitrogen. It should be placed between oxygen andcarbon.
(c) Beryllium < nitrogen < fluorine
(d) Fluorine (F)
(b) Nitrogen. It should be placed between oxygen andcarbon.
(c) Beryllium < nitrogen < fluorine
(d) Fluorine (F)
Question 2
A group of elements in the periodic table is given below ( Boron is the first member of the group and thallium is the last)
Boron
Aluminium
Gallium
Indium
Thallium
Answer the following questions in relation to the above group of elements:
(a) Which element has the most metallic character?
(b) Which element would be expected to have the highest electro negativity?
(c) If the electronic configuration of aluminium is 2, 8, 3, how many electrons will be there in the outermost shell of thallium?
(d) The atomic number of boron is 5. Write the chemical formula of the compound formed when boron reacts with chlorine.
(e) Will the elements in the group to the right of this boron group be more metallic or less metallic in character? Justify your answer.
Boron
Aluminium
Gallium
Indium
Thallium
Answer the following questions in relation to the above group of elements:
(a) Which element has the most metallic character?
(b) Which element would be expected to have the highest electro negativity?
(c) If the electronic configuration of aluminium is 2, 8, 3, how many electrons will be there in the outermost shell of thallium?
(d) The atomic number of boron is 5. Write the chemical formula of the compound formed when boron reacts with chlorine.
(e) Will the elements in the group to the right of this boron group be more metallic or less metallic in character? Justify your answer.
Solution 2
(a) Thallium
(b) Boron
(c) 3
(d) BCl3
(e) The elements in the group to the right of this boron group will be less metallic in character because on moving to the right of the periodic table metallic character decreases as ionization energy deceases and tendency to lose electron also decreases.
(b) Boron
(c) 3
(d) BCl3
(e) The elements in the group to the right of this boron group will be less metallic in character because on moving to the right of the periodic table metallic character decreases as ionization energy deceases and tendency to lose electron also decreases.
Question 3
Choose the correct answer:
With reference to the variation of properties in the Periodic Table, which of the following is generally true?
(a) Atomic size increases from left to right across a period.
(b) Ionization potential increases from left to right across a period.
(c) Electron affinity increases going down a group.
(d) Electro negativity increases going down a group.
With reference to the variation of properties in the Periodic Table, which of the following is generally true?
(a) Atomic size increases from left to right across a period.
(b) Ionization potential increases from left to right across a period.
(c) Electron affinity increases going down a group.
(d) Electro negativity increases going down a group.
Solution 3
(a) False
(b) True
(c) False
(d) True
(b) True
(c) False
(d) True
Question 4
The following questions refer to the Periodic Table.
(a) (i) Name the first and last element in period 2.
(ii) What happens to the atomic size of elements moving from top to bottom of a group?
(iii) Which of the elements has the greatest electron affinity among the halogens?
(iv) What is the common feature of the electronic configurations of the elements in group 7?
(b) Supply the missing word from those in the brackets:
(i) If an element has a low ionization energy then it is likely to be ______ (metallic/ non-metallic).
(ii) If an element has seven electrons in its outermost shell then it is likely to have the ______ (largest/ smallest) atomic size among all the elements in the same period.
(c) (i) The metals of group 2 from top to bottom are: Be, Mg, Ca, Sr, Ba. Which of these metals will form ions most readily and why?
(ii) What property of an element is measured by electro negativity?
(a) (i) Name the first and last element in period 2.
(ii) What happens to the atomic size of elements moving from top to bottom of a group?
(iii) Which of the elements has the greatest electron affinity among the halogens?
(iv) What is the common feature of the electronic configurations of the elements in group 7?
(b) Supply the missing word from those in the brackets:
(i) If an element has a low ionization energy then it is likely to be ______ (metallic/ non-metallic).
(ii) If an element has seven electrons in its outermost shell then it is likely to have the ______ (largest/ smallest) atomic size among all the elements in the same period.
(c) (i) The metals of group 2 from top to bottom are: Be, Mg, Ca, Sr, Ba. Which of these metals will form ions most readily and why?
(ii) What property of an element is measured by electro negativity?
Solution 4
(a) (i) First element in period 2 is Lithium and last element is Neon.
(ii)Atomic size increases on moving from top to bottom of a group.
(iii)Chlorine among halogens has the greatest electron affinity.
(iv) All elements in group 7 have same number of valence shell electrons.
(b) (i) metallic
(ii) smallest
(c) (i) Ba i.e. Barium will form ion most readily since it is at the bottom of a group its ionization energy is low because its atomic size is more. Due to this effective atomic charge of nucleus over the valence shell electron is least and it can be removed easily.
(ii) Electro negativity of an element measures the capacity of an element to attract the shared pair of electrons in a bond towards itself.
(ii)Atomic size increases on moving from top to bottom of a group.
(iii)Chlorine among halogens has the greatest electron affinity.
(iv) All elements in group 7 have same number of valence shell electrons.
(b) (i) metallic
(ii) smallest
(c) (i) Ba i.e. Barium will form ion most readily since it is at the bottom of a group its ionization energy is low because its atomic size is more. Due to this effective atomic charge of nucleus over the valence shell electron is least and it can be removed easily.
(ii) Electro negativity of an element measures the capacity of an element to attract the shared pair of electrons in a bond towards itself.
Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 22
Question 1
Choose the correct answer:
Among the period 2 elements the one which has high electron affinity is:
(a) Lithium
(b) Carbon
(c) Fluorine
(d) Neon
Among the period 2 elements the one which has high electron affinity is:
(a) Lithium
(b) Carbon
(c) Fluorine
(d) Neon
Solution 1
(d) Fluorine
Question 2
Solution 2
Question 3
an element has an atomic number 16. State
- the period to which it belongs
- the number of valence electrons
- whether it is a metal or non-metal
Solution 3
Atomic number of an element is 16.
- It belongs to Period 16.
- Number of valence electrons in the element is 6.
- The element is a non-metal.
Question 4
Give reasons as to why - The oxidising power of elements increases on moving from left to right along a period in periodic table
Solution 4
The oxidising power of elements depends on the tendency to gain electrons which increases from left to right along a period due to increase in nuclear pull.
Question 5
Give the number of the group and the period of elements having three shells with three electrons in valence shell.
Solution 5
Three shells indicate that the element belongs to the third period.
Three valence electrons indicate that the element belongs to the third group.
Three valence electrons indicate that the element belongs to the third group.
Question 6
Fill in the blanks from the choices given below
(i) Across a period, the ionisation potential ………… [increases, decreases, remains same]
(ii) Down the group, electron affinity ………… [increases, decreases, remains same]
Solution 6
- Across a period, the ionisation potential increases.
- Down the group, electron affinity decreases.
Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 23
Question 1
Choose the correct answer from the choice given:
(i) In the periodic table, alkali metals are placed in the group __________
(a) 1 (b) 11 (c) 17 (d) 18
(ii) Which of the following properties do not match with elements of the halogen family?(a) They have seven electrons in their valence shell.
(b)They are highly reactive chemically.
(c) They are metallic in nature.
(d) They are diatomic in their molecular form.
Solution 1
(i) In the periodic table, alkali metals are placed in Group I. So, the correct option is A.
The elements of halogen family are non-metallic in nature.
Question 2
Among the
Period 2 elements, the element which has high electron affinity is
- Lithium
- Carbon
- Chlorine
- Fluorine
Solution 2
Correct
option: (D) Fluorine
Question 3
Identify
the metallic oxide which is amphoteric in nature.
(a) Calcium oxide
(b) Barium oxide
(c) Zinc oxide
(d) Copper oxide
Solution 3
(c) Zinc oxide
Question 4
which element has the highest ionisation potential.
Solution 4
The element which has the highest ionisation potential is helium (He).
Question 5
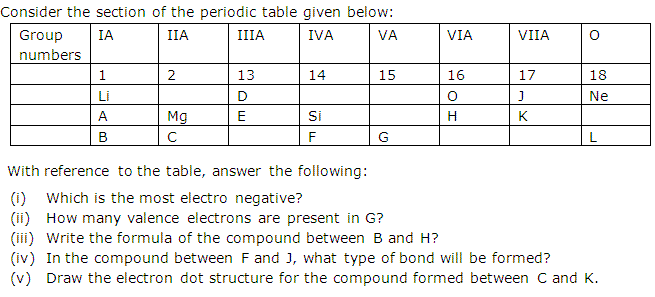
Group No.
|
IA
1
|
IIA
2
|
IIIA
13
|
IVA
14
|
VA
15
|
VIA
16
|
VIIA
17
|
0
18
|
2nd period
|
Li
|
D
|
O
|
J
|
Ne
|
|||
3rd period
|
A
|
Mg
|
E
|
Si
|
H
|
M
|
||
4th period
|
R
|
T
|
I
|
Q
|
U
|
Y
|
In the above table, H does not represent hydrogen.
Some elements are given in their own symbol and position in the periodic table
while others are shown with a letter.
With refrence to the table answer the following questions.
- Identify the most electronegative element.
- Identify the most reactive element of Group I.
- Identify the element from Period 3 with least atomic size.
- How many valence electrons are present in Q?
- Which element from group 2 would have the least ionisation energy?
- Identify the noble gas of the fourth period.
- In the compound between A and H, what type of bond would be formed and give its molecular formula.
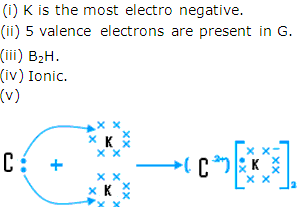
Solution 5
- I
- R
- M
- 5
- T
- Y
- Ionic bond will be formed and the molecular formula is A2H.
Question 6
Choose the correct answer from the choice given:
i. Ionisation potential increases over a period from left to right because the
- Atomic radius and nuclear charge increase
- Atomic radius and nuclear charge decrease
- Atomic radius increases and nuclear charge decreases
- Atomic radius decreases and nuclear charge increases
Solution 6
- Correct option: D (atomic radius decreases and nuclear charge increases)
Question 7
ii. An element A belonging to Period 3 and Group II will have
- 3 shells and 2 valence electrons
- 2 shells and 3 valence electrons
- 3 shells and 3 valence electrons
- 2 shells and 2 valence electrons
Solution 7
- Correct option: A (3 shells and 2 valence electrons)
Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 24
Question 1
An element Z has atomic number 16. Answer the following
(a) State the period and group to which Z belongs
(b) Is Z a metal or a non-metal?
(c) State the formula between Z and hydrogen.(d) what kind of a compund is this?
Solution 1
(a) An element Z having atomic number 16 is Sulphur.
(i) Sulphur belongs to Period 3 and Group 16.(ii) Sulphur is a non-metal.
(b) Two hydrogen atoms combine with one sulphur atom to form hydrogen sulphide (H2S) gas.
Question 2
Among
the elements given below, the element with the least electronegativity is :
(a) Lithium
(b) Carbon
(c) Boron
(d) Fluorine
Solution 2
(i) Lithium
Reason:
Electronegativity increases from left to right. Lithium is present on the
left side of the periodic table; hence, it will be the least electronegative
element.
Question 3
The
metals of Group 2 from top to bottom are Be, Mg, Ca, Sr and Ba.
(1) Which one of these elements will form ions
most readily and why?
(2) State the common feature in the electronic configuration
of all these elements
Solution 3
(1) Ba metal will form ions readily because the ionsation energy decreases down the group as the size
increases.
(2) On moving down the group, the number of electrons in
the outermost shell, i.e. valence electrons, remain the same. So, the valency
in the group remains the same.
Question 4
Arrange the following as per instructions given in the brackets.
a. Cs, Na, Li, K, Rb (decreasing electronegativity)
b.Mg, Cl, Na, S, Si (increasing order of atomic size)
c.Na, K, Cl, S, Si (increasing ionisation potential)
d.Cl, F, Br, I (increasing electron affinity)
Solution 4
a. Mg, Cl, Na, S, Si (increasing order of atomic size) -
Cl < S < Si < Mg < Na
99 pm < 104 pm < 117 pm < 160 pm < 186 pm
b. Cs, Na, Li, K, Rb (increasing metallic character)
Li < Na < K < Rb < Cs
c. Na, K, Cl, S, Si (increasing ionisation potential) -
Cl < S < Si < Na < K
1256 < 999 < 786 < 496 < 419
d. Cl, F, Br, I (increasing electron affinity) -
I < Br < F < Cl
-295 KJ mol-1 < -324 KJ mol-1 < -327.9 KJ mol-1 < -349 KJ mol-1
e. Cs, Na, Li, K, Rb (decreasing electronegativity) -
Li > Na > K = Rb > Cs
1.0 > 0.9 > 0.8 = 0.8 > 0.7
Question 5
Choose
the most appropriate answer from the following list of oxides which fit the
description. Each answer may be used only once: [SO2,SiO2,Al2O3,MgO,CO,Na2O]
(a) A basic oxide
(b) An oxide which dissolves in water forming
an acid.
(c) An amphoteric oxide
(d) A covalent oxide of a metalloid
Solution 5
(a) A basic oxide: Na2O (Na2O
+
H2O →
2NaOH)
(b) An oxide which dissolves in water forming
an acid: SO2 (SO2+ H2O → H2SO3)
(c) An amphoteric oxide: Al2O3 (shows
both acidic and basic properties)
(d) A covalent oxide of a metalloid: SiO2
(Si is a metalloid)
Question 6
Rewrite
the following sentences by using the correct > (greater than) or <
(less than) in the blanks given :
(i) The ionization potential of potassium is _______ that of sodium.
(ii) The electronegativity of iodine is ________ that of
chlorine.
Solution 6
(i) < (less than)
(ii) < (less than)
Question 7
Use
the letters only written in the Periodic Table given below to answer the
questions that follow:
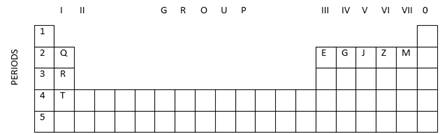
(a) State the number of valence electrons in
the atom J.
(b) Which element shown forms ions with a
single negative charge?
(c) Which Metallic element is more reactive than R?
(d) Which element has its electrons arranged in
four shells?
Solution 7
(a) The element J is placed in the 5th group;
hence, its number of valence electrons is 5.
(b) The element M which is placed in the 7th
group has 7 valence electrons and will gain only one electron to complete its
octet; hence, element M will show a single negative charge.
(c) Element T is more reactive than element R because
chemical reactivity increases down the group.
(d) Only element T is placed in the 4th period; hence,
its electrons are arranged in four shells.
Chapter 1 - Periodic Properties And Variation Of Properties: Physical And Chemical Exercise 25
Question 1
Fill
in the blanks by selecting the correct word from the brackets.
(a) If an element has a low ionization energy
then it is likely to be ________ (metallic/non-metallic)
(b) If an element has seven electrons in its
outermost shell then it is likely to have the_____ (largest/ smallest) atomic
size among all the elements in the same period.
Solution 1
(a) If an element has low ionisation energy,
then it is likely to be metallic.
(b) If an element has seven electrons in its outermost
shell, then it is likely to have the smallest atomic size among all
the elements in the same period.
Question 2
Fill
in the blank from the choice given in bracket.
(a) The energy required to remove on electron
from a natural isolated gaseous atom and convert it into a positively charged
gaseous ion is called ____________ (electron affinity, ionization potential,
electronegativity)
Solution 2
(a) The energy required to remove on electron from a
natural isolated gaseous atom and convert it into a positively charged
gaseous ion is called ionisation potential.
Question 3
Arrange
the following as per the instruction given in the brackets
(a) He,Ar,Ne (Increasing order of the number of
electron shells)
(b) Na,Li,K (Increasing ionization energy)
(c) F,Cl,Br (Increasing electronegativity)
(d) Na,K,Li (Increasing atomic size)
Solution 3
(a) He < Ne < Ar
(b) K < Na <
Li
(c) Br < Cl < F
(d) Li < Na < K
Question 4
Match
the atomic number 2,4,8,15, and 19 with each of the following :
(a) A solid non-metal belonging to the third
period
(b) A metal of valency 1.
(c) A gaseous element with valency 2.
(d) An element belonging to Group 2.
(e) A rare gas.
Solution 4
(a) A solid non-metal belonging to the third
period: 15
(b) A metal of valency 1: 19
(c) A gaseous element with valency 2: 8
(d) An element belonging to Group 2: 4
(e) A rare gas: 2

0 comments:
Post a Comment