Chapter 11F - Carboxylic acid Exercise 290
Question 1
Solution 1
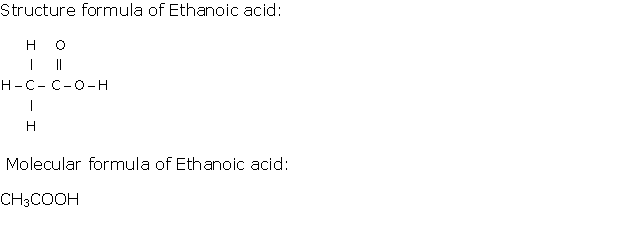
Question 2
Solution 2
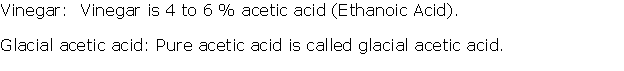
Question 3
Write three physical properties of acetic acid.
Solution 3
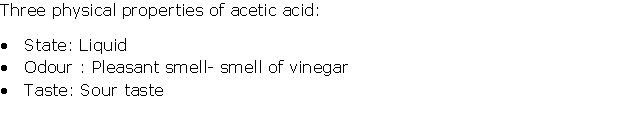
Question 4
Solution 4

Question 5
Solution 5
Question 6
What is the result of oxidation of ethyl alcohol?
Solution 6
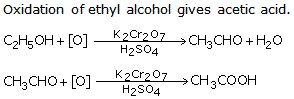
Question 7
Solution 7
Question 8
Solution 8
Question 9
Solution 9
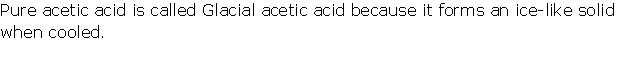
Question 10
Solution 10
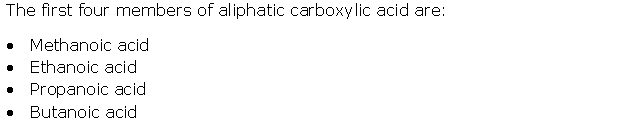
Question 11

Solution 11
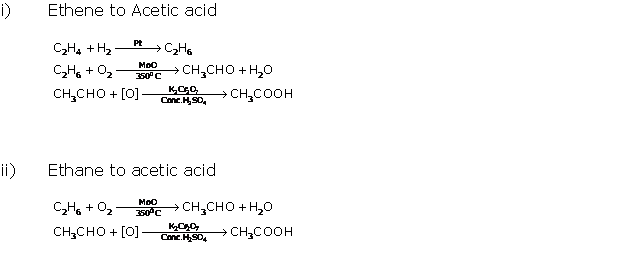
Question 12
Fill
in the blank with appropriate word/words.
(i) Vinegar is a solution of about ________per cent
________in water.
(ii) The general formula for alkene
and alkyne are _____ and______respectively
(iii) The catalyst used for hydrogen of vegetable oil is
___________
(iv) For artificial ripening of fruits _________is used
(v) Bayer's reagent is _________
(vi) Esterification is the reaction between carboxylic acid and ______ in
presence of ____
(vii) Denatured alcohol is a mixture of _____ and _______
(viii) For test of unsaturation
_____is used
(ix) In laboratory ethyne is
prepared by the reaction of water an _________
(x) Two isomers of the compound having molecular formula
C3H6O are __________and ________
Solution 12
(i) Vinegar is a solution of about 4 to 6
per cent acetic acid in water.
(ii) The general formula for alkene
and alkyne are CnH2n
and CnH2n-2,respectively.
(iii) The catalyst used for hydrogen of vegetable oil is nickel.
(iv) For artificial ripening of fruits, ethylene
is used.
(v) Bayer's reagent is alkaline KMnO4.
(vi) Esterification is the reaction between carboxylic acid and alcohol in the presence of conc.
H2SO4.
(vii) Denatured alcohol is a mixture of ethyl
alcohol and methanol.
(viii) For test of unsaturation,
bromine water is used.
(ix) In the laboratory, ethyne is
prepared by the reaction of water and calcium carbide.
(x) Two isomers of the compound having molecular formula C3H6O
are propanal
and propanol.
Question 13
(i) Compounds having the same molecular formula but
different properties are called
(a) Isotopes
(b) Isomers
(c) Isobars
(d) Allotrops
(ii) The first organic compound prepared by Wohler in the
laboratory was
(i) acetic acid
(ii) methane
(iii) Acetylene
(iv) Urea
(iii) Soda-lime is a mixture of
(a) NaOH + CaCl2
(b) NaOH + CaCO3
(c) NaOH + CaO
(d) NaOH + Al2O3
(iv) When sodium propionate is heated with soda-lime, the
product formed is
(a) Methane
(b) Ethane
(c) Ethene
(d) Ethyne
(v) LPG contains
(a) Methane
(b) Ethane
(c) Butane
(d) None of these
(vi) Methane reacts with energy of chlorine in diffused
sunlight to give the final product as
(a) Chloroform
(b) Carbon tetrachloride
(c) Methylene chloride
(d) Methyl chloride
(vii) Ethene reacts with 1% cold alkaline KMnO4 to
give
(a) Oxalic acid
(b) Ethyl alcohol
(c) Acetaldehyde
(d) Ethylene glycol
(viii) When ethanol is heated with conc. H2SO4
at 170oC, it gets converted into ethane. In this reaction conc. H2SO4
acts as
(a) Oxidizing agent
(b) Catalyst
(c) Dehydrating agent
(d) Reducing agent
Solution 13
(i) Isomers
(ii) Urea
(iii) NaOH + CaO
(iv) Ethane
(v) Butane
(vi) Carbon tetrachloride
(vii) Ethylene glycol
(viii) Dehydrating agent
Chapter 11F - Carboxylic acid Exercise 291
Question 1

Solution 1
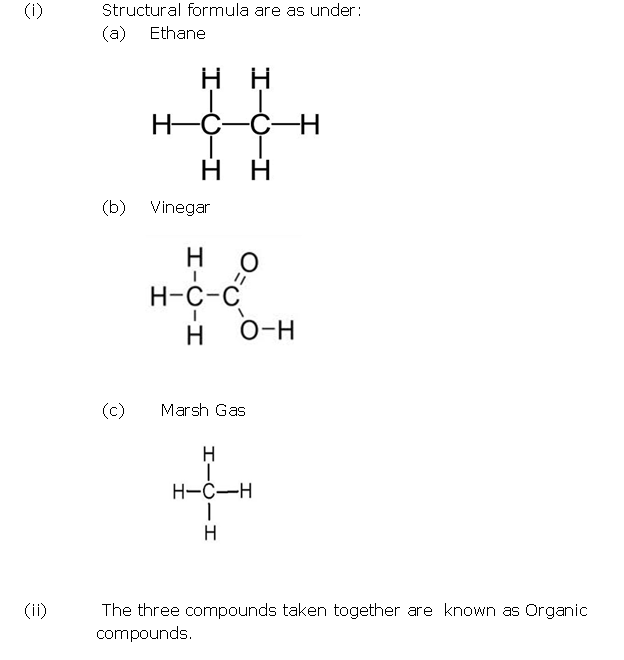
Question 2

Solution 2

Question 3
Solution 3
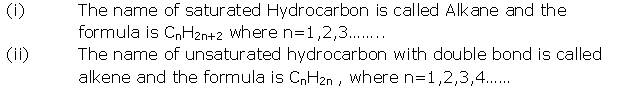
Question 4
Solution 4
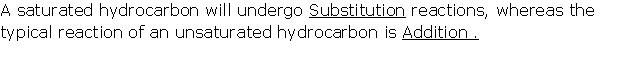
Question 5

Solution 5

Question 6
Solution 6

Question 7

Solution 7
Chapter 11F - Carboxylic acid Exercise 292
Question 1

Solution 1

Question 2
Write down the equation for the preparation of ethyne from calcium carbide?
Solution 2
Question 3
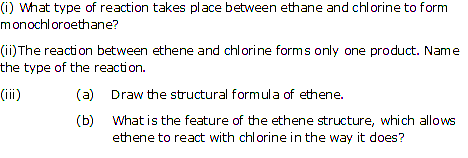
Solution 3
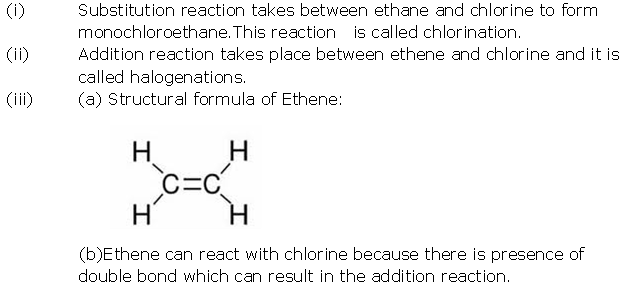
Question 4

Solution 4

Question 5

Solution 5
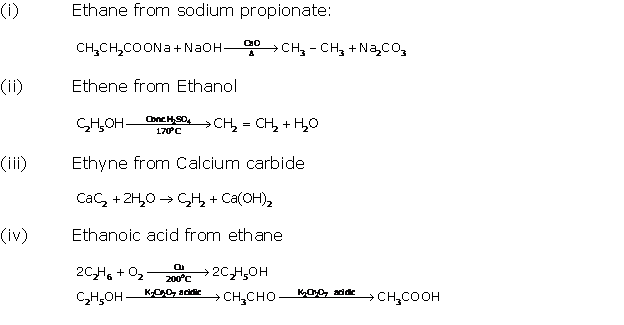
Question 6

Solution 6

Question 7

Solution 7

Chapter 11F - Carboxylic acid Exercise 293
Question 1

Solution 1
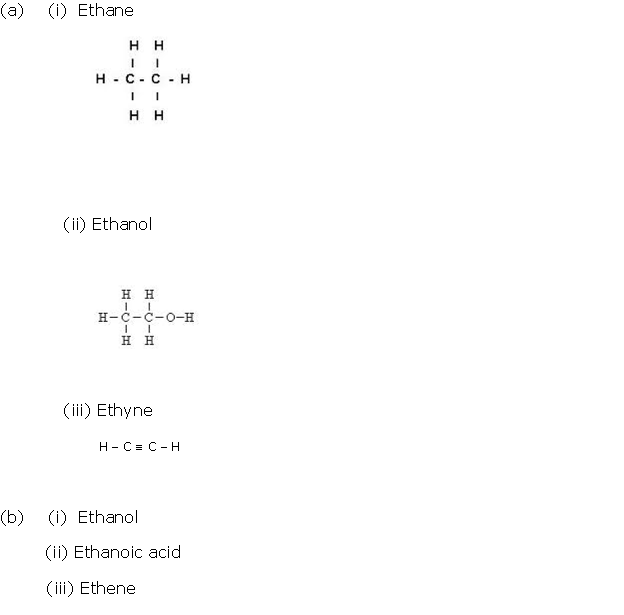
Question 2

Solution 2
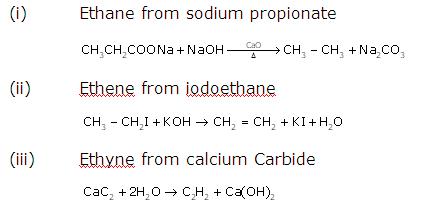
Question 3
Solution 3

Question 4
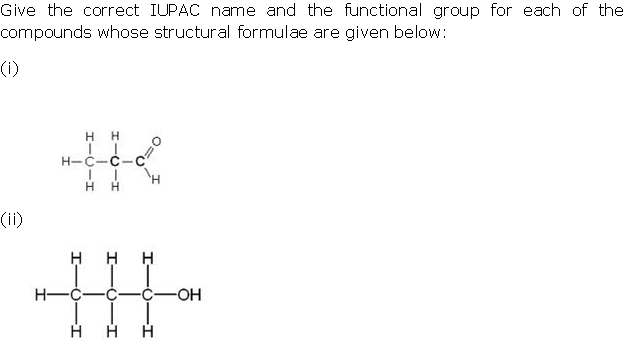
Solution 4

Question 5

Solution 5

Question 6

Solution 6

Question 7
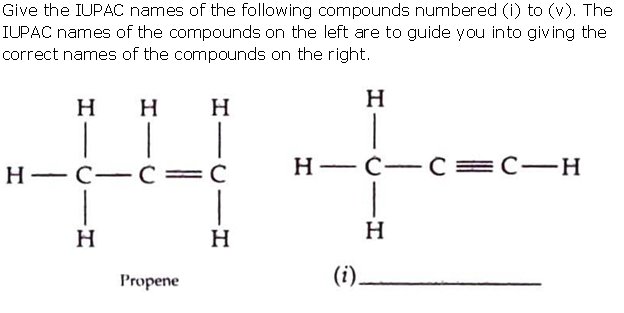
Solution 7

Chapter 11F - Carboxylic acid Exercise 294
Question 1
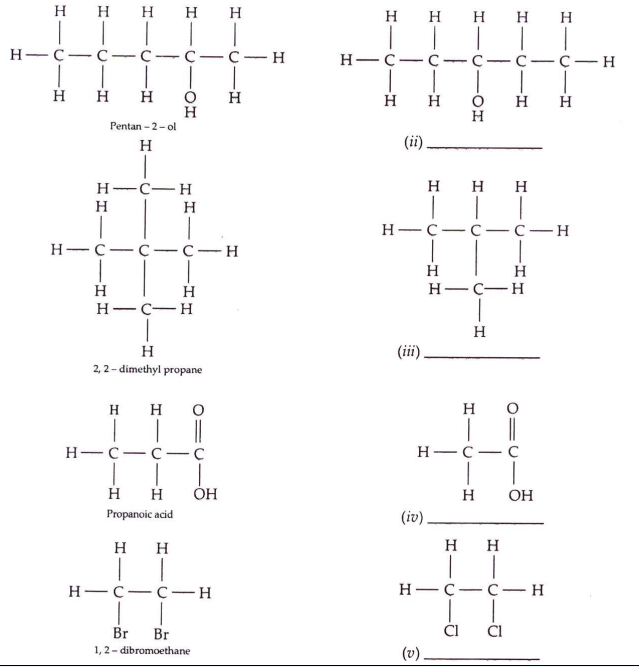
Solution 1
Question 2

Solution 2
Chapter 11F - Carboxylic acid Exercise 295
Question 1

Solution 1

Question 2

Solution 2
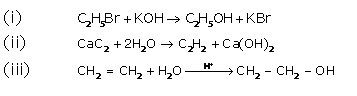
Question 3

Solution 3
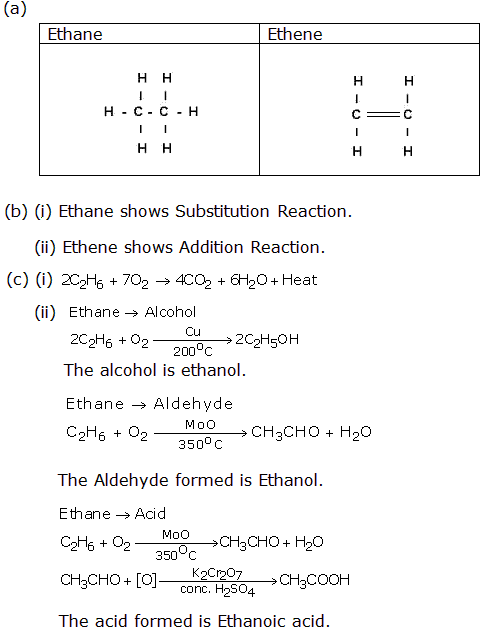
Question 4

Solution 4

Question 5
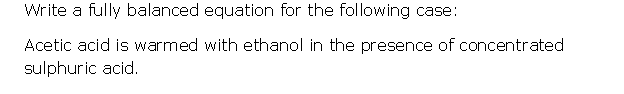
Solution 5
Question 6

Solution 6

Question 7

Solution 7
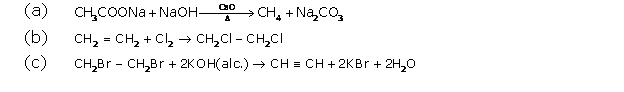
Chapter 11F - Carboxylic acid Exercise 296
Question 1

Solution 1
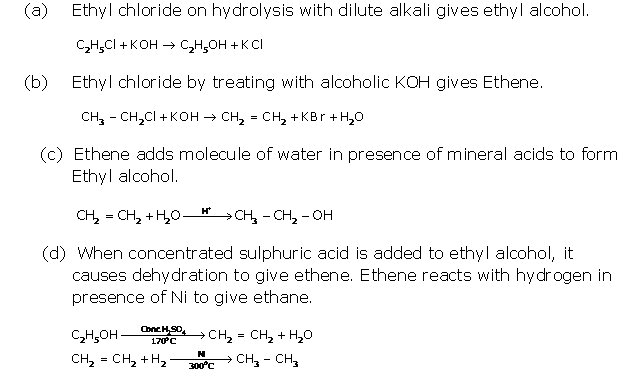
Question 2
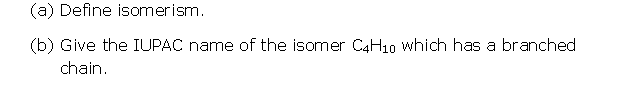
Solution 2
Question 3
Select
the correct answer from the choices a, b, c and d which are given.
(i) An organic compound undergoes addition reactions and
gives a red colour precipitate with ammonial cuprous chloride. Therefore, the organic
compound could be:
(a) Ethane
(b) Ethene
(c) Ethyne
(d) Ethanol
(ii) The organic compound mixed with ethanol to
make it spurious is :
(a) Methanol
(b) Methanoic acid
(c) Methanal
(d) Ethanoic acid
Solution 3
(i) (c) Ethyne
(ii) (a) Methanol
Question 4
Draw
the structural formula for each of the following:
(a) Ethanoic acid
(b) But-2-yne
Solution 4
(a)
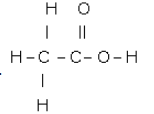
(b)
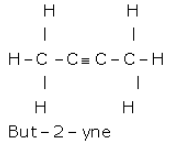
Question 5
Compound
A is bubbled through bromine dissolved in carbon tetrachloride and the
product is CH2Br - CH2Br.
(i) Draw the structural formula of A.
(ii) What type of reaction has A
undergone?
(iii) What is your observation
(iv) Name (not formula) the compound formed when steam
reacts with A in the presence of phosphoric acid.
(v) What the procedure for converting the product of (b) (IV)
back to A?
Solution 5
(i) H2C=CH2
(ii) Addition reaction
(iii) Bromine solution gets decolourised.
(iv) Ethanol
(v) By heating it (ethanol) with concentrated sulphuric acid at 170oC.
Question 6
Give
reason as to why :
(i) Almost 90% of all known compounds are organic in nature
(ii) It is dangerous to burn methane in an
insufficient supply of air.
Solution 6
(i) Carbon can form a large number of compounds
because of tetravalency and catenation.
(ii) In insufficient supply of air, methane burns
to produce carbon monoxide which is a toxic gas.
Question 7
Choose
the correct answer from the options given below :
(i) The number of C-H bonds in ethane molecule are :
(a) Four
(b) Six
(c) Eight
(d) Ten
(ii) The functional group present in acetic acid is :
(a) Ketonic (C = 0)
(b) Hydroxyl (- OH)
(c) Aldehydic (- CHO)
(d) Carboxyl (-COOH)
(iii) The unsaturated hydrocarbons undergo:
(a) A substitution reaction
(b) An oxidation reaction
(c) An addition reaction
(d) None of the above
Solution 7
(i) (b) Six
(ii) (d) Carboxyl (-COOH)
(iii) (c) An addition reaction
Question 8
Choose
the correct word/phrase from within the brackets to complete the following
sentences :
(i) The catalyst used for conversion of ethane to ethane is
commonly ________ {nickel/iron/cobalt)
(ii) When acetaldehyde us oxidized with acidified potassium
dichromate, it forms _________ (ester/ethanol/acetic acid)
(iii) Ethanoic acid reacts with ethanol in presence of concentrated H2SO4
so as to form a compound and water. The chemical reaction which takes place
is called ______ (dehydration/hydrogenation/esterification)
(iv) Write the equation for the reaction taking place
between 1, 2-dibromoethane and alcoholic potassium hydroxide.
(v) The product formed when ethane gas reacts with water in
the presence of sulphuric acid is __________ (ethanal/ethanol/ethanoic acid)
Solution 8
(i) Nickel
(ii) Acetic acid
(iii) Esterification
(iv) 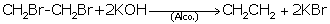
(v) Ethanol
Chapter 11F - Carboxylic acid Exercise 297
Question 1
Write
balanced chemical equation for the following :
(i) Monochloroethane is hydrolysed with aqueous
KOH
(ii) A mixture of sodalime and
sodium acetate is heated
(iii) Ethanol under high pressure and low temperature is
treated with acidified potassium dichoromate
(iv) Water is added to calcium carbide
(v) Ethanol reacts with sodium at room temperature
Solution 1
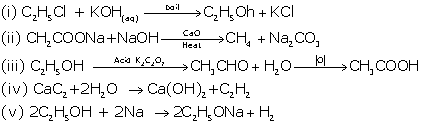
Question 2
Give
the structural formulae for the following :
(i) An isomer of n-butane
(ii) 2-propanol
(iii) Diethyl ether
Solution 2
(i) An isomer of n-butane
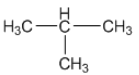
(ii) 2-propanol
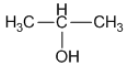
(iii)Diethyl ether
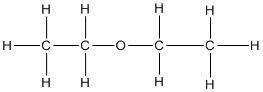
Question 3
Give
reasons for the following :
(i) Methane does not undergo addition reaction, but ethane
does
(ii) Ethyne is more reactive than ethane
(iii) Hydrocarbons are excellent fuels
Solution 3
Reasons:
(i) Alkanes are
saturated, i.e. they do not have a carbon-to-carbon double bond, and so do
not undergo an addition reaction. Alkenes are said to be unsaturated because
of the carbon-to-carbon double bond in their structure, and the double bond
in alkenes makes them more reactive than alkanes.
(ii) Ethyne is a
highly reactive compound due to the presence of a triple bond between its two
carbon atoms.
(iii) Hydrocarbons are used as fuels because they
burn in air producing a lot of heat energy.
Question 4
Give
balanced equation for the laboratory preparations of the following organic
compounds:
(i) A saturated hydrocarbon from iodomethene
(ii) An unsaturated hydrocarbon from an alcohol
(iii) An unsaturated hydrocarbon from calcium carbide
(iv) An alcohol from ethyl bromide
Solution 4
Equations:
(i) CH3I + 2[H] → CH4 + HI
Iodo Methane
(ii) CH3CH2OH + H2SO4→ CH3CH2HSO4
+ H2O
CH3CH2HSO4→ CH2=CH2
+ H2SO4
(iii)CaC2
+ 2H2O → Ca(OH)2
+ C2H2↑
(iv) C2H5Br + NaOH → C2H5OH
+ NaBr
Question 5
Identify
this statement that is incorrect about alkanes.
(a) They are hydrocarbons
(b) There is a single covalent bond between
carbon and hydrogen
(c) They can undergo both substitution as well as addition
reactions
(d) On complete combustion they produce carbon
dioxide and water
Solution 5
(c) They can undergo both substitution as well as addition reactions.
Question 6
(a) Identify the gas evolved when sodium
propionate is heated with sodalime
(b) Give a chemical term for a reaction in
which hydrogen of a alkane is replaced by a halogen
Solution 6
(a) Ethane
(b) Substitution
Question 7
Give
a chemical test to distinguish between ethane gas and ethane gas.
Solution 7
Ethene gas decolourises the purple colour
of KMnO4, whereas ethane does not decolourise
KMnO4 solution.
Question 8
Give
balanced equation for the labortary preparation of
the following organic compounds:
(a) A saturated hydrocarbon from iodomethene
(b) An unsaturated hydrocarbon from an alcohol
(c) An unsaturdated hydrocarbon
from calcium carbide
(d) An alcohol from ethyl bromide
Solution 8
Equations:
(i) CH3I + 2[H] → CH4 + HI
(ii) CH3CH2OH
+ H2SO4→ CH3CH2HSO4 + H2O
CH3CH2HSO4→ CH2=CH2 + H2SO4
(iii) CaC2 + 2H2O
→ Ca(OH)2 + C2H2↑
(iv) C2H5Br
+ NaOH → C2H5OH
+ NaBr
Chapter 11F - Carboxylic acid Exercise 298
Question 1
Give
the structural formula of the following
(i) Ethanol
(ii) 1-propanal
(iii) Ethanoic acid
(iv) 1,2,dichlormethene
Solution 1
(i) Ethanol: CH3-CH2-OH
(ii)
1-propanal
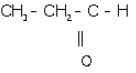
(iii) Ethanoic acid
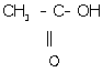
(iv) 1,2, dichloromethene
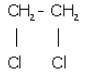
Question 2
The
IUPAC name of acetylene is
(a) Propane
(b) Propyne
(c) Ethane
(d) ethyne
Solution 2
(d) ethyne
Question 3
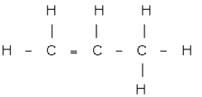
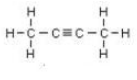
Write
the IUPAC names of each of the following :
(i)
(ii)
(iii)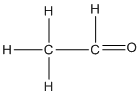

Solution 3
(a) Propene
(b) But-1-yne
(c) Ethane
Question 4
Using
their structural formulae identify the functional group by circling them :
(i) Dimethyl ether
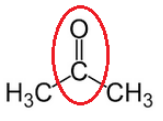
(ii) propanone
Solution 4
(i) Dimethyl ether
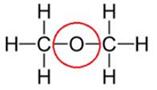
(ii) Propanone
Question 5
Identify
the statement which does not describe the property of alkenes:
(a) They are unsaturated hydrocarbons
(b) They decolourise
bromine water
(c) They can undergo addition as well as substitution
reactions
(d) They undergo combustion with oxygen forming
carbon dioxide and water.
Solution 5
(c) They can undergo addition as
well as substitution reactions.
Question 6
State
the observation
When
the gaseous product obtained by dehydration of ethyl alcohol is passed
through bromine water.
Solution 6
The
reddish brown colour of bromine solution gets decolourised.
Question 7
Name
the following :
(i) Process by which ethane is obtained from ethane
(ii) A hydrocarbon which contributes towards the greenhouse
effect
(iii) Distinctive reaction that takes place when ethanol is treated
with acetic acid
(iv) The property of elements by virtue of which atoms of
the element can link to each other in the form of a long chain or ring structure
Solution 7
(i) Hydrogenation
(ii) Methane
(iii) Esterification
(iv) Catenation
Question 8
Give
balanced chemical equations for the following conversions :
(i) Ethanoic acid to ethyl ethanote
(ii) Calcium carbide to ethyne
(iii) Sodium ethanoate to methane
Solution 8
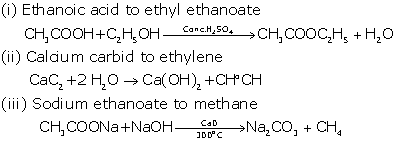
Chapter 11F - Carboxylic acid Exercise 299
Question 1
Give
the structural formulae of each of the following :
(i) 2-methyl propane
(ii) Ethanoic acid
(iii) Butna-2ol
Solution 1
(i) 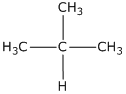

(ii) 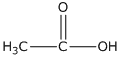

(iii) 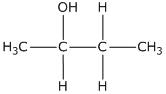

Question 2
Equation
for that reaction when compound A is bubbled through bromine dissolved in
carbon trtrachloride is as follows :
DIAGRAM
(i) Draw the structure of A.
(ii) State your observation during this reaction
Solution 2
(i) 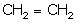
(ii) When bromine
dissolved in CCl4 is added to ethene,
the orange colour of bromine disappears because of the formation of
colourless ethylene bromide.
Question 3
Write
a balanced chemical equation for each of the following:
(i) Burning of ethane in plentiful supply for air
(ii) Action of water on calcium carbide
(iii) Heating of ethanol at 170oC in the presence
of conc. Sulphuric acid
Solution 3
(i) 
(ii) 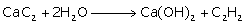
(iii) 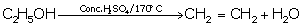
Question 4
Choose
the correct answer from the options given below :
If
the molecular formula of an organic compouns is C10H18
it is
(a) Alkene
(b) Alkane
(c) Alkyne
(d) Not a hydrocarbon
Solution 4
(3) alkyne
Alkyne has the general molecular formula CnH2n-2.
Question 5
Fill
in the blank.
The
compound formed when ethane reacts with hydrogen is _________ (CH4,C2H6,C3H6).
Solution 5
The compound formed when ethane reacts
with hydrogen is C2H6.
Question 6
Identify
the underlined substance.
An
organic compound containing -COOH functional group.
Solution 6
An
organic compound containing -COOH functional group: carboxylic
acid
Question 7
State
are relevant observations for following reactant:
Addition
of ethyl alcohol to acetic acid in presence of conc. H2SO4
Solution 7
On
addition of ethyl alcohol to acetic acid in the presence of conc. H2SO4
at high temperature, sweet smelling ethyl acetate ester is produced, and the
process is known as esterification.
C2H5OH
+ CH3COOH 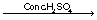 CH3COOC2H5
+ H2O
CH3COOC2H5
+ H2O
Question 8
Draw
the structural formula for each of the following :
(i) 2, 3-dimethyl butane
(ii) Diethyl ether
(iii) Propanoic acid
Solution 8
(i) 2, 3-dimethyl butane
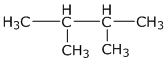
(ii) diethyl ether
(iii)propanoic acid
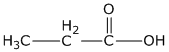
Question 9
Identify
the term or substance based on the descriptions given below:
(i) Ice like crystals formed on cooling an organic acid
sufficiently.
(ii) Hydrocarbon containing a triple bond used for welding purposes
(iii) The property by virtue of which the compound has the
same molecular formula but different structural formulae.
(iv) The compound formed where two alkyl are linked by
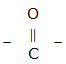
group
Solution 9
(i) Glacial
acetic acid
(ii) Acetylene
(iii) Isomerism
(iv) Ketones
Question 10
Give
a balanced chemical equation for each of the following :
(i) Preparation of ethane from sodium propionate
(ii) Action of alcoholic KOH on bromoethane
Solution 10
(i) 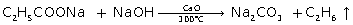
(ii) 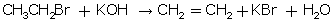

0 comments:
Post a Comment