Chapter 2 - Study of Gas Laws Exercise 21
Question 1
Write the name and symbol of each state variable of a gas.
Solution 1
An ideal gas can be characterized by three state variables:
(i) Absolute pressure (P),
(ii) Volume (V), and
(iii) Absolute temperature (T).
(i) Absolute pressure (P),
(ii) Volume (V), and
(iii) Absolute temperature (T).
Question 2
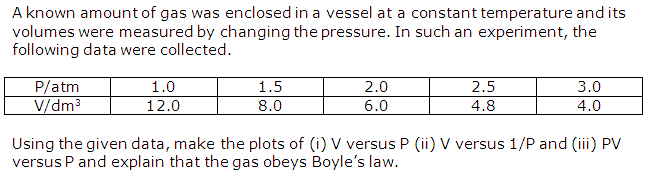
Solution 2
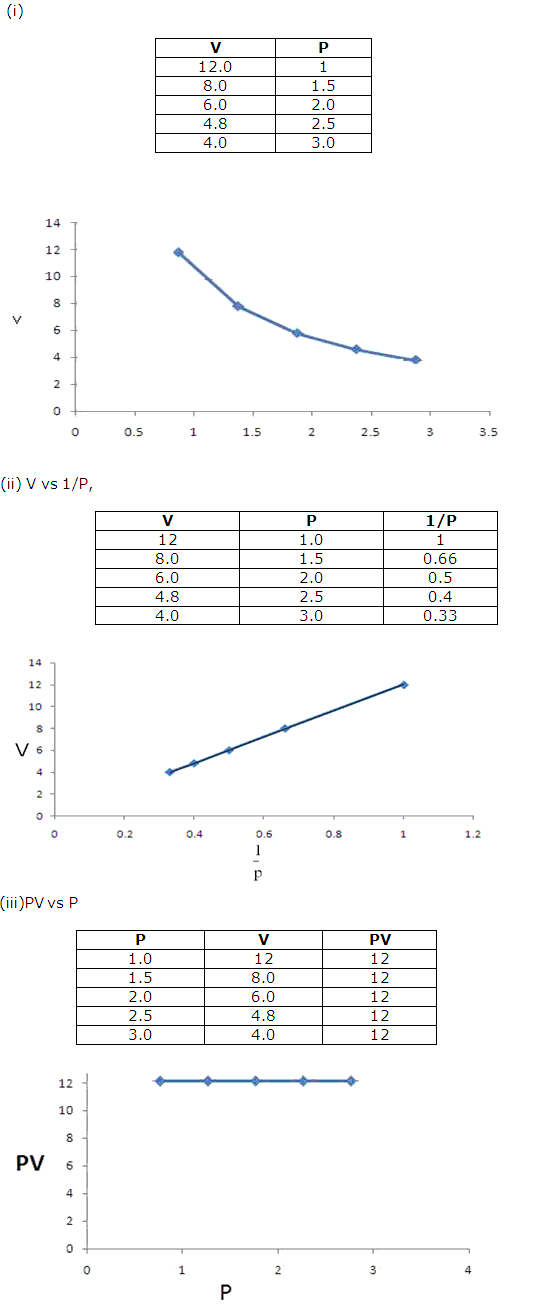
Question 3
Solution 3
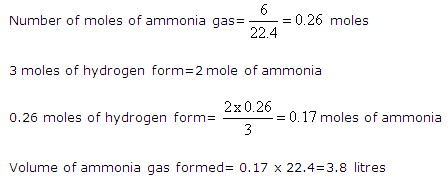
Question 4
When stating the volume of a gas the pressure and temperature should also be given. Why?
Solution 4
There is simultaneous effect of temperature and pressure changes on the volume of a given mass of a gas. So, when stating the volume of a gas, the pressure and temperature should also be given.
Question 5
State Boyle's law.
Solution 5

Question 6
Deduce a relationship between volume and pressure for a given mass of a gas at a constant temperature.
Solution 6
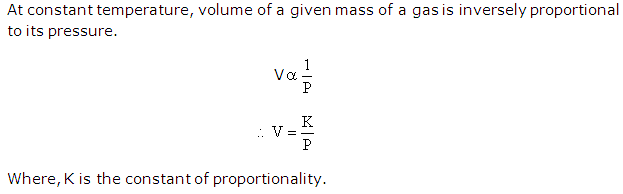
Question 7
State Charles' law.
Solution 7
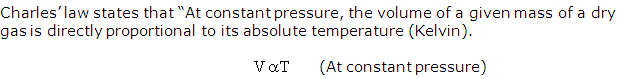
Question 8
Write the value of Kelvin zero.
Solution 8
Kelvin zero is -273.15oC.
Question 9
Write the mathematical statements of Boyles' law and Charles' law. Use these statements to derive the combined gas equation.
Solution 9

Question 10
Define STP.
Solution 10
The standard temperature and pressure (STP) by general convention are 0OC(273 K) and 1 atm(760 mm Hg).
Question 11
(a) Write the value of standard temperature in (i) oC and (ii) K
(b) Write the value of standard pressure in (i) atm, (ii) mm Hg, (iii) cm Hg, (iv) torr
(b) Write the value of standard pressure in (i) atm, (ii) mm Hg, (iii) cm Hg, (iv) torr
Solution 11
(a) The value of standard temperature is (i) 0oC and (ii) 273 K
(b)The value of standard pressure is (i) 1 atm, (ii) 760 mm of Hg, (iii)76 cm of Hg, (iv)760 torr
(b)The value of standard pressure is (i) 1 atm, (ii) 760 mm of Hg, (iii)76 cm of Hg, (iv)760 torr
Question 12
A sample of gas occupies 45.6 cm3 at 240 mm Hg pressure and 25oC . What volume will it occupy at 720 mm Hg?
Solution 12
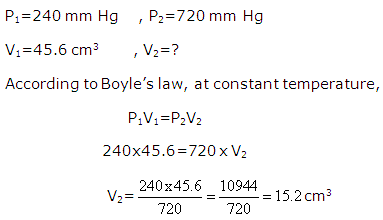
Chapter 2 - Study of Gas Laws Exercise 22
Question 1
Is it possible to change the temperature and pressure of a fixed mass of a gas without changing its volume? Explain your answer.
Solution 1
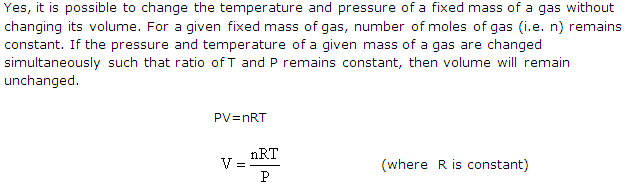
Question 2
Explain briefly:
(a) The Kelvin scale has been adopted for chemical calculation.
(b) The absolute zero is a theoretical concept.
(c) When stating the volume of a gas, the pressure and temperature should also be given.
(a) The Kelvin scale has been adopted for chemical calculation.
(b) The absolute zero is a theoretical concept.
(c) When stating the volume of a gas, the pressure and temperature should also be given.
Solution 2
(a) Volume of a gas would be reduced to zero at 0 K (-2730C).All temperatures on the Kelvin scale are positive, so Kelvin scale has been adopted for chemical calculation.
(b) At absolute zero temperature, volume of a gas would be reduced to zero. Theoretically,this is the lowest temperature that can be reached. At this temperature all molecular motions cease. Thus, practically this temperature is impossible to attain because on cooling gases liquefy and Charles' law is no more applicable.
(c) According to combined gas law equation, there is simultaneous effect of temperature and pressure changes on the volume of a given mass of a gas. So, when stating the volume of a gas, the pressure and temperature should also be given.
(b) At absolute zero temperature, volume of a gas would be reduced to zero. Theoretically,this is the lowest temperature that can be reached. At this temperature all molecular motions cease. Thus, practically this temperature is impossible to attain because on cooling gases liquefy and Charles' law is no more applicable.
(c) According to combined gas law equation, there is simultaneous effect of temperature and pressure changes on the volume of a given mass of a gas. So, when stating the volume of a gas, the pressure and temperature should also be given.
Question 3
4 litres of CO2 is kept at 270C.Calculate its volume if the volume is lowered to 150K at the same pressure.
Solution 3

Question 4
A gas cylinder containing cooking gas can withstand a pressure of 14.9 atmosphere. The pressure gauge of the cylinder indicates 12 atmospheres at 270C.Due to sudden fire in the building, its temperature starts rising. At what temperature will the cylinder explode?
Solution 4
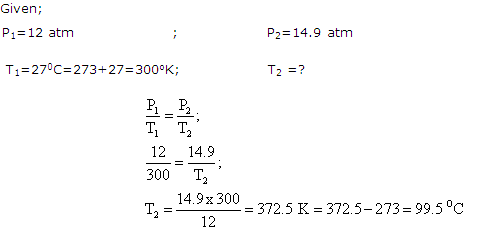
Question 5
At 0oC and 760 mm Hg pressure, a gas occupies a volume of 100 cm3.The Kelvin temperature of the gas is increased by one fifth while the pressure is increased by one and a half times. Calculate the final volume of the gas.
Solution 5
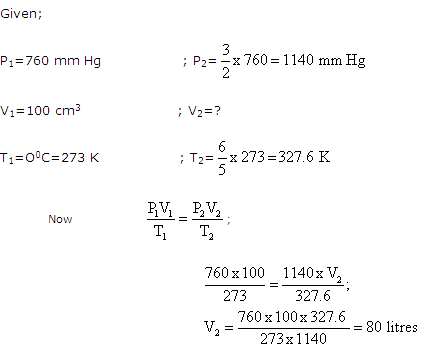
Question 6
Which of the following statements are true and which are false?
(a) At constant pressure if a sample of a gas is heated from 0Oc to 2730C,the volume will be double.
(b) At constant pressure if a sample of a gas is heated from 4750C TO 12730C,the volume will be double.
(c) Boyle studied the variation of the volume of a gas with pressure and found that as the pressure increases, so does the volume.
(d) A gas exerts pressure at the bottom but not on the walls of container.
(e) The P-V graph of a fixed mass of a gas at constant temperature is a straight line.
(a) At constant pressure if a sample of a gas is heated from 0Oc to 2730C,the volume will be double.
(b) At constant pressure if a sample of a gas is heated from 4750C TO 12730C,the volume will be double.
(c) Boyle studied the variation of the volume of a gas with pressure and found that as the pressure increases, so does the volume.
(d) A gas exerts pressure at the bottom but not on the walls of container.
(e) The P-V graph of a fixed mass of a gas at constant temperature is a straight line.
Solution 6
(a) True
(b) False
(c) False
(d) False
(e) False
(b) False
(c) False
(d) False
(e) False
Question 7
How can you explain Boyle's law and Charles' law on the basis of the kinetic theory?
Solution 7
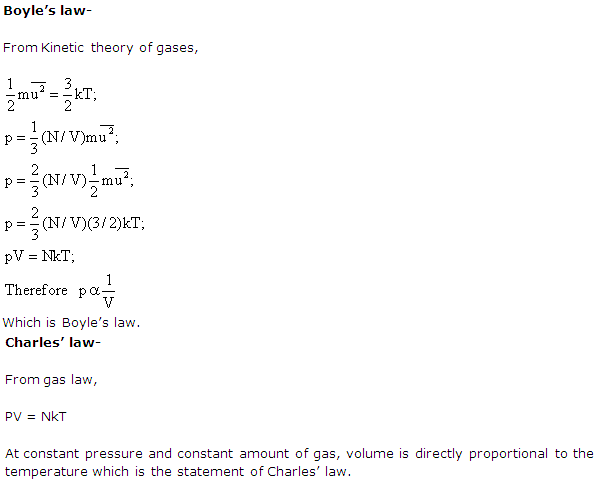
Question 8
A sample of methane occupies 320 ml at 47oC.To what temperature (in degree celsius) will it have to be heated to occupy 450 ml at the same pressure?
Solution 8
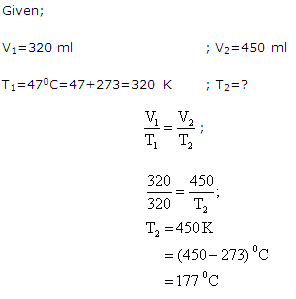
Question 9
Give an experiment to show the pressure volume relationship of gas.
Solution 9
We trap a definite quantity of air in the closed vessel. At any point, the pressure on the air is equal to the atmospheric pressure plus the pressure due to the excess mercury column in the open end tube. By pouring mercury in the tube, we increase the pressure on the air and measure its volume under that pressure. We thus obtain a set of data for the volume of a fixed mass of air under different pressures.
For a given mass of air at constant temperature, the following observations are made-
(i) The volume of air decreases with increasing pressure and vice versa.
(ii) The proportion by which the volume decreases or increases is the same by which the pressure increases or decreases.
For a given mass of air at constant temperature, the following observations are made-
(i) The volume of air decreases with increasing pressure and vice versa.
(ii) The proportion by which the volume decreases or increases is the same by which the pressure increases or decreases.
Question 10
The molecular theory accounts for the pressure exerted by a gas in a closed vessel as a result of the gas molecules striking against the wall of the vessel. How will the pressure change if :
(a) The temperature is doubled keeping the volume constant,
(b) The volume is halved, keeping the temperature constant?
(a) The temperature is doubled keeping the volume constant,
(b) The volume is halved, keeping the temperature constant?
Solution 10
(a) Pressure will also be doubled.
(b) Pressure will be double.
(b) Pressure will be double.
Question 11
Fill in the blanks:
(a) The melting point of the ice is ______ Kelvin.
(b) The temperature on the Kelvin scale at which molecular motion completely ceases is called ______.
(c) The average kinetic energy of the molecule of a gas is proportional to the ______.
(d) If temperature is reduced to one half, ______ would also reduced to one half.
(a) The melting point of the ice is ______ Kelvin.
(b) The temperature on the Kelvin scale at which molecular motion completely ceases is called ______.
(c) The average kinetic energy of the molecule of a gas is proportional to the ______.
(d) If temperature is reduced to one half, ______ would also reduced to one half.
Solution 11
(a) 273
(b) absolute zero
(c) absolute temperature
(d) the average kinetic energy
(b) absolute zero
(c) absolute temperature
(d) the average kinetic energy
Question 12
2 litres of a gas is enclosed in a vessel when the pressure is 760 mm Hg. If the temperature remains constant, calculate the pressure when:
(a) the volume changes to 4 dm3,
(b) the volume is increased by one and half times.
(a) the volume changes to 4 dm3,
(b) the volume is increased by one and half times.
Solution 12
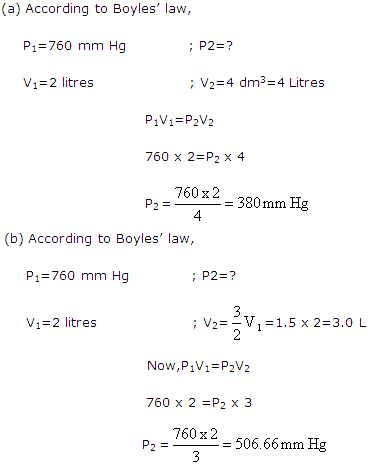
Question 13
Calculate the volume of dry air at STP that occupies 28 cm3 at 140C and 750 mm Hg pressure when saturated with water vapour. The vapour pressure of water at 140C is 12 mm Hg.
Solution 13
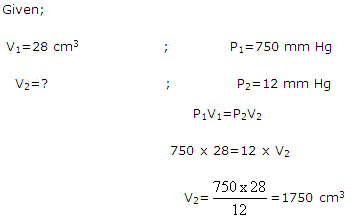
Question 14
A given amount of a gas X is confined in a chamber at constant volume. When the chamber is immersed in a bath of melting ice, the pressure of the gas is 100 cm Hg.
(a) What is the temperature, when the pressure is 10 cm of Hg?
(b) What will be the pressure when the chamber is brought to 100 oC?
(a) What is the temperature, when the pressure is 10 cm of Hg?
(b) What will be the pressure when the chamber is brought to 100 oC?
Solution 14
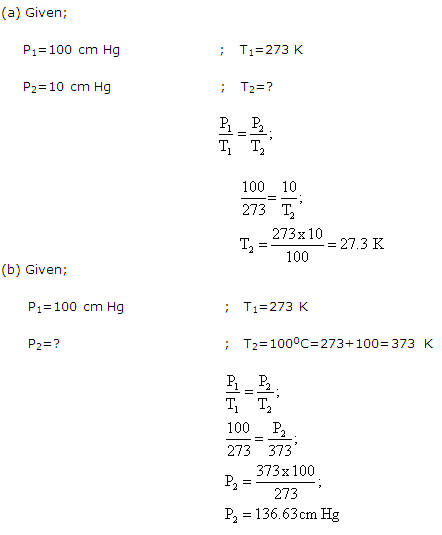
Question 15
A fixed mass of a gas has a volume of 750 cm3 at -23oC and 800 mm pressure. Calculate the pressure for which the volume will be 720 cm3, the temperature being -30C.
Solution 15
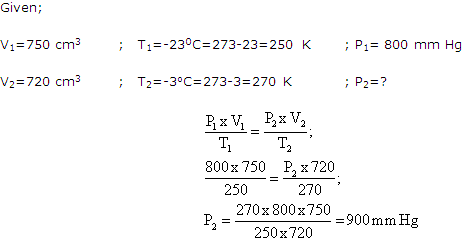
Question 16
Why does the size of a weather balloon become larger and larger as it ascends into higher space?
Solution 16
As weather balloon go higher into the atmosphere, the air becomes less dense, so air pressure drops. Because of this, the air that is already inside the balloon expands to cope with the difference in pressure. The end result is that the balloon expands making it larger.
Question 17
The pressure of a gas maintained in a steel tank is 20 atmospheres. If the pressure has to be increased by 20%, to what temperature must the tank be heated, if the initial temperature is 27oC?
Solution 17

0 comments:
Post a Comment